Volume 8 Issue 4
What’s Happening at Missouri S&T:
(formerly UMR)
Short Course Dates
We will be offering "Basic Composition of Coatings" March 26-30, 2012 (Spring 2012). The Basic Composition course is intended for new personnel in the coatings profession. It targets the components of coatings (resin, pigments, extenders, solvents and additives), testing and specifications, general formulation and manufacturing methods. Basic Composition is primarily a lecture course with several laboratory demonstrations.
We will be offering "Introduction to Paint Formulation" June 4-8, 2012 (Spring 2012). This course is intended to give the person a fundamental knowledge of how to approach a starting formulation and troubleshoot it. This course involves both lecture and laboratory work.
We will be offering "Introduction to Coatings Composition and Specifications" July 18-20, 2012 (Summer 2012), course designed for the new coatings person in areas such as sales, marketing or production. The course was initiated by a number of raw material companies and distributors requesting a course with this format. This course is not as heavily technical as is our “Basic Composition of Coatings" and “Introduction to Paint Formulation" courses. The "Introduction to Coatings Composition and Specifications" course is a two and a half day course which will discuss the types of coatings, the basic composition of coatings and the tests and specifications used by the industry. This course will allow the participant to gain the fundamentals needed to work in this industry and to communicate more clearly.
For more information see our web site at http://coatings.mst.edu/index.html and to register contact Catherine Hancock at cemv26@mst.edu or coatings@mst.edu or call 573-341-4419. **These courses are held on the Rolla Campus**
We have expanded our web operations, and we need your help in spreading the word. We are inviting people from industry and academia to join the Missouri S&T Coatings Institute Listserv. By joining our listserve, you'll be able to receive newsletter from us which has Missouri S&T coatings short courses dates, employment advertisements and short articles related to polymers and coatings. Please reply us at svgwcc@mst.edu with your name, company name and e-mail address to get added to our Listserv.
Employment Tab
We have started an employment section for our students and companies. We have a full time job section, an intern / co-op section and a graduating and alumni students section . Please explore our section on employment on our web site. Anyone wanting to have job opening listed, please contact us at (573) 341-4419 or e-mail: svgwcc@mst.edu . You can also write to us at Missouri S&T Coatings Institute, BOM #2, 651 W. 13th St., Rolla, MO 65409-1020. Our web site is http://coatings.mst.edu
Selection Criteria for Pigmented UV Cure Coatings
Sagar Gade, Graduate Research Student, Missouri S&T
During the ‘Introduction to paint formulation’ short course at Missouri S&T Coatings Institute, I work with the groups doing UV coatings. Many people form industry talk to us about recent developments in UV and EB curing coatings and the problems they face during formulating pigmented UV curing coatings. In this short abstract we will discuss the problems faced while formulating pigmented UV curing coatings and some recommendations to avoid them.
UV curing coatings being 100% solids (most of the time) and rapid curing, are popular in the coating industry. UV clear coatings are fairly easy to formulate and cure out well using the correct photoinitiator (PI) , UV source and monomer/oligomers for the desired set of properties. Thin pigmented coatings (especially UV curable black printing inks) also cure fairly quickly. But it is a real challenge to formulate pigmented coatings with high PVC, good curing and adhesion properties or thicker pigmented finishes. There are several monomers/oligomers, PI, pigments and UV sources available for curing. The following are selection guidelines for raw materials for better performance and curing properties.
Pigments: Many pigments including TiO2 absorb the UV part of radiation spectrum and therefore interfere with curing mechanism. Thick pigmented UV curable coating cures on the surface but through curing does not takes place as UV radiation fails to penetrate and therefore film delamination occurs. Below is the reflectance spectrum of TiO2 which absorb all the UV-B and UV-C radiation and major part of UV-A.
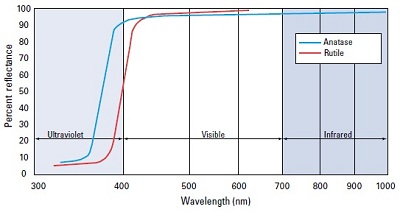 |
Fig 1. Reflectance curve of TiO2 Pigment1
In UV cure coatings, pigments compete with PI in both, UV and visible region considering absorption. Therefore it’s really important to reduce the overlap between the absorption spectra of the pigments and PIs to get an effective curing window. Knowing the absorption spectrum of pigments helps in the selection of the best PI. Penetration of radiation also depend on particle size of pigment, courser particles generally give better penetration.2
Solvents: UV curable systems are generally 100% solid system. Incorporation of pigment increases the viscosity of coating. Depending on application technique, if solvent is required to reduce viscosity, sufficient flash time must be given prior to coating entering the UV chamber. Solvent entrap in the cured coating result in softer films and can result in a variety of surface defects and problems. In general fast solvents are therefore preferred. Note that if methyl acetate or acetone or any exempt solvent is used, a zero VOC coating is still possible but many are flammable and can result in a fire hazard issue.
Photoinitiator, dispersion and level of loading: There are many types of PIs available on the market. Most of them work well with clear coats, which is not the case for pigmented coatings. In the past 15 years, with advancement in technology, a new class of PIs are available which dissociate to form free radicals at a wide spectral range (320 nm to 460 nm) these are called UV-Vis PI [(v) & (vi) in figure 2] and are commonly used commercially in pigmented UV systems. A combination of two or more PIs also improves curing, as dissociation occurs at wide spectrum of UV radiation.
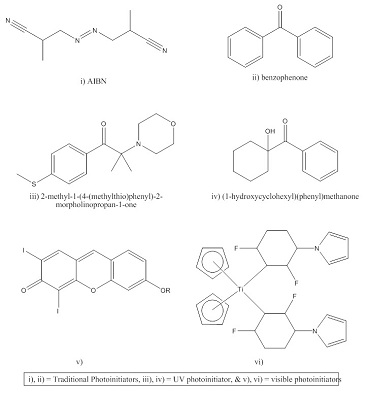 |
Fig 2. Different types of photoinitiators2,3
A wide range of PIs are available, as liquid or solid materials. Liquid PIs disperse very efficiently and can be added anytime during formulation. In the case of solid PIs, they are preferably dissolved in monomers/oligomers to disperse homogeneously throughout the coating prior to adding pigments. Presence of solid PI agglomerates give localized curing and hence gives inferior properties.
Selection of the PI is very important and one should try many different PIs at different loading levels and optimize it. At low loading level with short exposure time, some monomers/oligomers remain unreacted, also at high loading level a low molecular weight polymer forms which produces inferior mechanical properties. Optimization of PI loading level is important and is influenced by resin chemistry. ¿-max for decomposition to form free radicals, film thickness, pigment volume concentration, exposure time and UV source configuration will all influence the performance properties of final coating.
UV source and Exposure time: Choosing the UV emitting light source and choosing PI depend on each other. Bulbs with different spectral output are used to match chemistry and cure speeds. Choosing correct UV source which emits high intensity at the ¿-max for given PI is desirable for rapid curing of coating in short time. Common UV sources with different emission spectrum are available listed below.
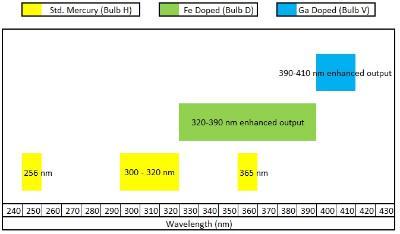 |
Fig.3 emission spectrum for UV bulb3
Warm up time, generally 5 to 15 minutes depending on lamp, should be provided to get stabilized radiation spectrum to get better curing rates. Intensity of radiation output from the UV lamp declines over a period of time thus limiting its lifetime. By monitoring emission spectrum using a radiometer and replacing lamps accordingly gives fast reproducible curing times. UV lamps do generate heat which causes evaporation of toxic monomers, therefore heat sink and ventilation must be checked in timely manner. Classic electrode discharge tubes produce significant IR and visible light which cause heating of the substrate. Use of the more modern microwave generated UV reduces the heating of the substrate.
UV LED and lasers are also available which emits radiation in narrow band or single wavelength. They give advantage of long source life, no intensity degradation and low heat generation. At the same time only selective PI can be used with these types of systems.4
References:
- http://www2.dupont.com/Titanium_Technologies/en_US/tech_info/literature/Coatings/CO_B_H_65969_Coatings_Brochure.pdf , Date: 11/21/2011
- http://www.rit.edu/~w-ue/courses/0305-676/reference/Imprint/Photoinitiators%20for%20UV%20curing.pdf , Date 11/29/2011
- Joseph V. Koleske, Radiation Curing of Coatings, ASTM Stock Number MNL45, 2002, West Conshohocken, PA, pp24
- http://www.ellsworth.com/display/displayFile.aspx?docid=1265&filename=/Glue_Doc_University_Curriculum/UV_Presentation.pdf , Date 11/29/2011
