Volume 6 Issue 3
What’s Happening at Missouri S&T:
(formerly UMR)
Spring Short Course Dates
This spring we will be offering ?Basic Composition of Coatings" March 29 to April 2, 2010. The Basic Composition course is intended for new personnel in the coatings profession. It targets the components of coatings (resin, pigments, extenders, solvents and additives), testing and specifications, general formulation and manufacturing methods. Basic Composition is primarily a lecture course with several laboratory demonstrations. For more information see our web site at http://coatings.mst.edu/index.html and to register contact Michael Van De Mark at coatings@mst.edu or call 573-341-4419. **This course is held on the Rolla campus**
This spring we will be offering ?Introduction to Paint Formulation" May 17-21, 2010. This course is intended to give the person a fundamental knowledge of how to approach a starting formulation and troubleshoot it. This course involves both lecture and laboratory work. For more information see our web site at http://coatings.mst.edu/index.html and to register contact Michael Van De Mark at coatings@mst.edu or call 573-341-4419. **This course is held on the Rolla campus**
Summer 2010 Short Course - "Introduction to Coatings Composition and Specifications"
** Register Today - Some Spaces Still Available!!!**
This summer we will be offering "Introduction to Coatings Composition and Specifications" July 19-21, 2010 in St. Louis, MO, a course designed for the new coatings person in areas such as sales, marketing or production. The course was initiated by a number of raw material companies and distributors requesting a course with this format. This course is not as heavily technical as is our “Basic Composition of Coatings" and “Introduction to Paint Formulation" courses. The ?Introduction to Coatings Composition and Specifications" course is a two and a half day course which will discuss the types of coatings, the basic composition of coatings and the tests and specifications used by the industry. This course will allow the participant to gain the fundamentals needed to work in this industry and to communicate more clearly. More information can be found at the above links, on our website at http://coatings.mst.edu/index.html, by emailing coatings@mst.edu or by calling 573-341-4419.
Technical Insights on Coatings Science
Review : ATR-IR Spectroscopy for the Coatings Industry
Jigar Mistry, Graduate Research Student, Missouri S&T
Introduction :
Spectroscopy is a technique that uses absorption, emission, or scattering of electromagnetic radiation by matter to perform qualitative or quantitative analysis of the matter.
Attenuated total reflection infrared (ATR-IR) spectroscopy is a subset of Infrared spectroscopy which enables samples to be examined directly in the solid or liquid state without any preparation. ATR spectroscopy and multiple internal reflection spectroscopy are increasingly gaining popularity as significant tools to analyze coatings and opaque liquids.
Principles of ATR :
A beam of infrared light is passed through the ATR crystal which is covered on the top by the solid or the liquid sample such that the incident infrared light reflects internally after coming in contact with the crystal covered with the sample, forming an evanescent wave which extends into the sample, typically by a few microns. The angle of incidence must be greater than the critical angle for total internal reflectance of the infrared light. When the beam exits the crystal, it is collected by a detector and analyzed and displayed in form of the ATR-IR spectra. The ATR Spectra is simply the IR Spectra, so all the typical uses apply.
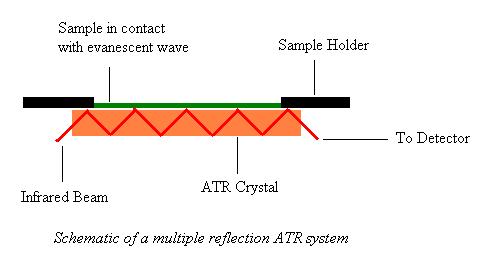 |
Instrumentation :
Any IR or FT-IR spectrometer can be used for ATR-IR
ATR Crystals :
ZnSe (Zinc Selenide), Thallium Bromide, Thallium Iodide and Germanium crystals are commonly used and are low in cost but they scratch easily and Thallium compounds are toxic. Diamond, being durable and robust, is by far the best ATR crystal material, but the original cost is obviously very high.
Properties of infrared transmitting materials for ATR-IR spectroscopy :
Material | Comments | Short Wavelength (cm-1) | Long Wavelength (cm-1) | Refractive Index | pH Range |
ZnSe | Most common | 15000 | 461 | 2.40 | 5-9 |
Diamond | Very costly | 30000 | <2 | 2.40 | 1-14 |
Ge | Brittle | 5500 | 432 | 4.0 | 1-14 |
Thallium Bromide / Thallium Iodide | Very toxic | 17900 | 204 | 2.37 | 5-8 |
Use of ATR-IR spectroscopy in the Coatings Industry :
The ATR-IR spectroscopy has been used for the chain orientation, functional group analysis, curing rate, oxidation studies, characterization of unsaturation and crystallinity studies of polymers. They have also been used for the quantitative analysis of resin components. The use of ATR for the quantitative determination of o-phthalic and isophthalic acids, vinyl toluene and styrene has been reported by Harris [1]. ATR-IR has also been used for the characterization of pigments used in the paintings [2], the effect of pigments on UV-degradation of epoxy systems [3], analysis of corrosion characteristics of coatings [4], etc. The chemical analyses of the surface of the polymer systems [5-10] and the pigment [11-13], separately, have been made using ATR -FTIR and XPS.
Conclusion :
ATR provides excellent quality data along with reproducibility with the advantage of faster sampling and minimizing the user-to-user spectral variation and can be put to a variety of uses for solid and liquid sampling of various components and aspects of a coating. Modern systems allow multi-scan averaging which increases the utility and applicability to many coatings problems.
References :
1. Harris, R. L., Svoboda, G. R., Anal. Chem., 34, 1655 (1962)
2 Applied Surface Science 255 (2009) 5172–5176
3 Proceedings of the 81st Annual Meeting Technical Program of the FSCT,
November 13-November 14, 2003, Pennsylvania Convention Center, Philadelphia, PA
4 H. Yun et al., Electrochimica Acta 52 (2007) 6679–6685
5. Dilks, A. “Surface Degradation of Polymers Studied by ESCA”, Degradation and Stability of Polymers, Vol. 1, Jellinek, H.H.G., Ed., Elsevier: Amsterdam, 601-628, (1983).
6. Clark, D.T. “Synthesis, Characterization, Modification, and Degradation of Polymer Surfaces as Revealed by ESCA”, Pure Appl. Chem, 57, 941, (1985).
7. Hawkridge, A.M., Gardella, Jr., J.A., Toselli, M. “ Investigation of the Water-Induced
Reorganization of Polycaprolactone-Poly(fluoroalkylene oxide)-Polycaprolactone Triblock Copolymer Films by Angle Dependent X-ray Photoelectron Spectroscopy”, Macromolecules, 35, 6533, (2002).
8. Motyakin, M.V., Schlick, S. “Thermal Degradation at 393K of Poly(acrylonitrile-butadienestyrene) (ABS) Containing a Hindered Amine Stabilizer: A Study by 1D and 2D Electron Spin Resonance Imaging (ESRI) and ATR-FTIR”, Polym. Degrad. Stab., 76, 25, (2002).
9. Haverkamp, R.G., Siew, D.C.W., Barton, T.F. “ XPS Study of the Changes During the Service Life of Polyester Powder Coatings”, Surf. Interface Anal., 33, 330, (2002).
10. Walters, K.B., Schwark, D.W., Hirt, D.E. “Surface Characterization of Linear Low-Density Polyethylene Films Modified with Fluorinated Additives”, Langmuir, 19, 5851, (2003).
11. Egerton, T.A., Parfitt, G.D., Kang, Y., Wightman, J.P. “XPS Analysis of Uncoated and Silica-Coated Titanium Dioxide Powders”, Colloids and Surf., 7, 311, (1983).
12. Diebold, U., Madey, T.E. “TiO2 by XPS” Surf. Sci. Spectra, 4, 227, (1997).
13. Erdem, B., Hunsicker, R.A., Simmsons, G.W., Sudol, E.D., Dimonie, V.L., El-Asser, M.S. “XPS and FTIR Surface Characterization of TiO2 Particles Used in Polymer Encapsulation”, Langmuir, 17, 2664, (2001).
14 http://en.wikipedia.org/wiki/Attenuated_total_reflectance (accessed December1, 2009)
15. http://www.piketech.com/technical/crystal-selection-transmission.html (accessed December1, 2009)
