Volume 5 Issue 1
What’s Happening at Missouri S&T:
(formerly UMR)
Summer 2008 Short Course - "Introduction to Coatings Composition and Specifications"
** Register Today - Some Spaces Still Available!!!**
This summer we will be offering "Introduction to Coatings Composition and Specifications" July 23-25, 2008, in Rolla, Missouri, a course designed for the new coatings person in areas such as sales, marketing or production. The course was initiated by a number of raw material companies and distributors requesting a course with this format. This course is not as heavily technical as is our “Basic Composition of Coatings" and “Introduction to Paint Formulation" courses. The ?Introduction to Coatings Composition and Specifications" course is a two and a half day course which will discuss the types of coatings, the basic composition of coatings and the tests and specifications used by the industry. This course will allow the participant to gain the fundamentals needed to work in this industry and to communicate more clearly. More information can be found at the above links, on our website at http://coatings.mst.edu/index.html, by emailing coatings@mst.edu or by calling 573-341-4419.
Fall Short Course Dates
This fall we will be offering ?Basic Composition of Coatings" September 15-19, 2008. The Basic Composition course is intended for new personnel in the coatings profession. It targets the components of coatings (resin, pigments, extenders, solvents and additives), testing and specifications, general formulation and manufacturing methods. Basic Composition is primarily a lecture course with several laboratory demonstrations. For more information see our web site at http://coatings.mst.edu/index.html and to register contact Michael Van De Mark at coatings@mst.edu or call 573-341-4419. **This course is held on the Rolla campus**
This fall we will be offering ?Introduction to Paint Formulation" November 3-7, 2008. This course is intended to give the person a fundamental knowledge of how to approach a starting formulation and troubleshoot it. This course involves both lecture and laboratory work. For more information see our web site at http://coatings.mst.edu/index.html and to register contact Michael Van De Mark at coatings@mst.edu or call 573-341-4419. **This course is held on the Rolla campus**
Lester A. Weinert Recieves ACS 50 Year Service Award
Mr. Lester A. Weinert received his bachelor degree in Chemistry at Iowa State University in 1952 and was then employed at Swifton Company in Tennessee. He joined the ACS in 1959. He then left Swifton to join Beckman Laboratories and worked in this company for 21 years. He left Buckman Labs to start Weinert Manufacturing Company. In 1998, he had sold the operation. In 2008, he started a new venture called "Grab Em Paints" in Louisburg, MO. Mr. Weinert has also lectured in the polymer and coatings short courses for a number of years at Missouri S&T alongside with Drs. Jim Stoffer, Michael Van De Mark and Harvest L. Collier. His area of specialty is in anti-corrosive and anti-microbial additives in coatings. For his continued passion and dedication in Chemistry, the South Central Missouri Local Section is pleased to present Mr. Lester A. Weinert with his Certificate of Recognition of 50 years of service to the American Chemical Society.
 |
| Lester Wienert Receiving ACS 50 Year Award |
Technical Insights on Coatings Science
Epoxy Powder Coatings
By Boonta Hetayothin, Graduate Student, Missouri S&T
Introduction
Powder coatings consist of pigment, additives and curing agent dispersed in a resin. The mixture is dried and ground into a fine powder. Powder coatings are actually melt mixed in an extruder after an initial dry mix. The extrudate is pressed in a set of chilled rollers and the resultant thin strip of material is cooled further on a belt before being chipped. The chips are then ground to the desired particle size to produce the final product. The coating is done by an electrostatic spray gun where the charged particles form a thin adherent layer on the substrate. Then it is heated so the powder particles are fused, coalesce and crosslink to give a tough, durable and insoluble coating [1].
Dry powder coatings technology dates back in 1950’s. It is used for finishing metal products, pipe and rebar coatings for corrosion protection and electrical parts for insulation. After the introduction of electrostatic spray process, the increased use of powder coatings as an alternative to solvent-based coatings has increased since 1970’s due to the increased concerns over VOC emissions (see Table 1), work safety and energy costs [2].
Powder coatings have several advantages such as being solvent free, easy to use without mixing, thinning and high recovery compared with the solvent-based paint. However, there are still some disadvantages, such as contamination of one powder with another, color change is not a rapid operation and tinting cannot be adjusted on site like in a wet paint.
Epoxy Resins
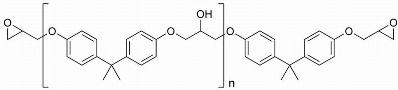
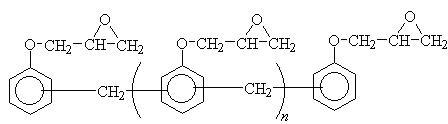
Epoxy powder coatings are one of the oldest and largest classes of thermosetting powder coatings. These resins are based on bisphenol A and novolac epoxies as shown in Figure 1.
Synthesis of epoxy resins first started in 1930’s. It was not until the 1950’s that they were taken into industrial use in protective coatings. The DGEBA resins are dominant in decorative coatings, which provide attractive finishes that are tough, corrosion resistant, flexible, and have high impact strength. The novolacs are more widely used for functional coatings and include corrosion resistance and outstanding electrical insulation. The multiplicity of the epoxy groups on epoxy-novolac resins allows higher crosslink density, which improves chemical, solvent and temperature resistance properties [1,3].
 |
(a)
 |
(b)
Figure 1.Epoxies (a) diglycidyl ethers of bisphenol A (DGEBA) and (b) epoxy-novolac resins.
One drawback of the aromatic epoxy polymers is their tendency to become yellow and degrade (chalk) upon UV exposure. For outdoor durable coatings, polyester TGIC (triglycidyl isocyanurate) or polyester urethane powder coatings are generally used. Aliphatic epoxy resins, for example glycidyl ethers based on hydrogenated bisphenol A, are sometimes considered for outdoor applications instead [3]. Epoxy-polyester hybrid coatings, which consist of epoxy and polyester resins, are used mainly for decorative interior applications. They are more resistant to chalking and yellowing than epoxies, but have a lower surface hardness and are less solvent resistant.
Curing agents and curing reactions
Curing agents have an importance influence on the properties of powder coatings, production, storage and application. Ideally they should be compatible with a resin having a similar melting point and efficiently react with the resin at the desired curing temperature. An epoxy group can react either anionically or cationically. Therefore curing agents can be classified as basic or acidic.
Basic curing agents include Lewis bases, secondary amines and primary amines. Acidic curing agents include Lewis acids, phenols, organic acids and anhydrides. However, common primary and secondary aromatic as well as aliphatic amines are far too reactive with epoxy resins to be used in powder coatings. Typical curing agents for epoxy powder coatings include dicyandiamide (DICY), 2-methylimidazole, cyclic amidines, phenolic-based hardeners, and acid-functional resins. See Figure 2.
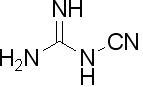 |
(a) dicyandiamide (DICY)
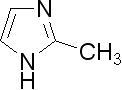 |
| (b) 2-methylimidazole |
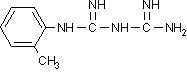 |
| (c) substituted DICY (o-toluyl biguanide) |
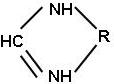 |
| (d) cyclic amidine |
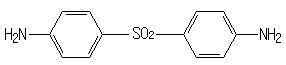 |
(e) diaminodiphenyl sulfone
Figure 2. Chemical structure of amine curing agents for epoxy powder coatings
Amine curing agents
I. Dicyandiamide-based
a). Dicyandiamide (DICY) is a well known curing agent for epoxy powder coatings. The curing reaction occurs through all four nitrogen-containing functional groups [4,5] but the mechanism is complex and not completely understood. However, the reaction of DICY with epoxy resin is too slow for practical use, unless catalyzed with a catalyst such as 2-methyl imidazole.
b). 2-methylimidazole (2-MI) is a widely used catalyst for DICY and other epoxy curing agents[6] c). Substituted DICY derivatives offer low melting point and improved compatibility with the epoxy resin although reactivity may not be as good as 2-MI.
II. Cyclic amidines
Cyclic amidines can be used to produce low temperature curing powders and amidine derivatives are good for low gloss finishes.
a). Low temperature curing amidine derivatives are highly reactive and epoxy powder curing can be done at temperatures as low as 120ºC. These may also be used to accelerate polyester-epoxy systems.
b). Amidine derivatives designed to produce low gloss are based on amidine salts of polycarboxylic acids. Low gloss coatings can be obtained if the right amount is used with epoxy resins.
III. Diaminodiphenyl sulfone
Glycidyl ethers of phenol-hydrocarbon novolacs are cured with diaminodiphenyl sulfone to obtain a better high temperature performance.
IV. Phenolic-based curing agents
Curing agents based on epoxy resins terminated with bisphenol A, or novolac resins, catalyzed with 2-MI or other catalyst.
V. Acid –functional resins
These include acid-functional polyester or acrylic resins, which are used with epoxy resins to form hybrids.
Table 1a. VOC Reduction Comparison (Metric Units) [2]
| Conventional solvent | Water borne | Higher solids | Powder |
Volume solids at spray viscosity, percentb | 33 | 35 | 60 | 99 |
Volume VOC content, percentc, d | 67 | 16 | 40 | 1 |
Actual coverage, m2/L (m2/kg for powder)b, e | 5.71 | 6.65 | 14.0 | 19.7 |
VOC emissions, metric tons/ yrf | 34.5 | 23.6 | 28.1 | 0.54 |
a Assumed 1.1x106 m2 of parts coated per year.
b Average of values presented in [2]
c Assumed density of solvent equals 882 g/L
d Water-borned coating VOC content assumed to be 25 percent of the nonsolids portion.
e Based on transfer efficiencies presented in [2]
f Control device assumed for conventional solvent coatings with overall efficiency of about 70 percent (based on capture efficiency of about 75 percent and destruction efficiency of about 95 percent). All other systems assumed to have no control device.
Conclusions
Significant improvement in powder coatings, the application and powder recovery system have made powder coatings one of the most cost effective and environmental friendly finishing systems available. Powder coatings are recognized as the lowest VOC content coatings among other available options for industrial finishers. Epoxy resin-based systems are among the most widely used thermosetting powders. (This is only true if consider all epoxy containing systems such as polyester-epoxy hybrids and polyester-TGIC) They are used for both decorative and functional coatings. The most commonly used hardeners or curing agents for epoxy resins in powder coatings include dicyandiamide (DICY), 2-methylimidazole (2-MI), cyclic amidines, phenolic-based hardeners, and acid-functional resins.
References
1. Howell, D.M. Powder Coatings: The Technology, Formulation and Application of Powder Coatings, John Wiley & Sons, New York, 2000.
2. Hester, C.I., Nicholson, R.L. and Cassidy, M.A. Powder Coating Technology, Noyes Data Corporation, Park Ridge, New Jersey, 1990.
3. Narkis, M. and Rosenzweig, N. Polymer Powder Technology, John Wiley & Sons, New York, 1995.
4. Sheik, A.Z. Advanced in organic coatings Science and Technology Series, 1982, 4, 83-102.
5. Sacher, E. Polymer., 1973, 14(3), 91-95.
6. Wicks, Z.W., Jones, F.N., Pappas, P.S. and Wicks, D.A. Organic Coatings: Science and Technology, 3rd Edition, John Wiley & Sons, New Jersey, 2007.
