Volume 9 issue 3
What’s Happening at Missouri S&T:
(formerly UMR)
Short Course Dates
We will be offering "Basic Composition of Coatings" March 25-29, 2013 (Spring 2013). The Basic Composition course is intended for new personnel in the coatings profession. It targets the components of coatings (resin, pigments, extenders, solvents and additives), testing and specifications, general formulation and manufacturing methods. Basic Composition is primarily a lecture course with several laboratory demonstrations.
We will be offering "Introduction to Paint Formulation" May 6-10, 2013(Spring 2013) . This course is intended to give the person a fundamental knowledge of how to approach a starting formulation and troubleshoot it. This course involves both lecture and laboratory work.
We will be offering "Introduction to Coatings Composition and Specifications" July 17-19, 2013 (Summer 2013), course designed for the new coatings person in areas such as sales, marketing or production. The course was initiated by a number of raw material companies and distributors requesting a course with this format. This course is not as heavily technical as is our “Basic Composition of Coatings" and “Introduction to Paint Formulation" courses. The ?Introduction to Coatings Composition and Specifications" course is a two and a half day course which will discuss the types of coatings, the basic composition of coatings and the tests and specifications used by the industry. This course will allow the participant to gain the fundamentals needed to work in this industry and to communicate more clearly.
For more information see our web site at http://coatings.mst.edu and to register contact Catherine Hancock at cemv26@mst.edu or coatings@mst.edu or call 573-341-4419. **These courses are held on the Rolla Campus**
Employment Tab
Don't forget to check our employment section for our students and companies. We have a full time job section, an intern / co-op section and a graduating and alumni students section . Please explore our section on employment on our web site. Anyone wanting to have job opening listed, please contact us at (573) 341-4419 or e-mail: Sagar Gade at svgwcc@mst.edu . You can also write to us at Missouri S&T Coatings Institute, BOM #2, 651 W. 13th St., Rolla, MO 65409-1020.
Technical Insights on coatings Science
Organic Corrosion Inhibitors: Factors that affect the inhibition efficiency of organic molecules
Yousef Dawib, Graduate Research Student
Missouri S&T Coatings Institute, Department of Chemistry
Introduction:
Due to environment regulation the use of chromate-based inhibitors has been limited, and great demands of alternative inhibitors have risen up. Organic corrosion inhibitors can be considered as a realistic alternative approach to inorganic inhibitors that can provide long term corrosion protection. Organic inhibitors can adsorb on metal surface and form a protective barrier that blocks the active dissolution sites of metal.
When metals become in contact with an aqueous environment, oxidation of metal begins at a high rate. Metal atoms are removed from their lattice with loss of the electrons to produce metal cations. Eventually, a dynamic equilibrium is attained and oxidation process terminates as a result of negative charge build up on metal surface. Polar water molecule will be attracted to the negatively charged metal surface to form ionic structure layer. This structure behaves essentially as a capacitor and prevents other ions from bulk solution to be part of it (1).
The following figure shows a schematic electrical double-layer model.
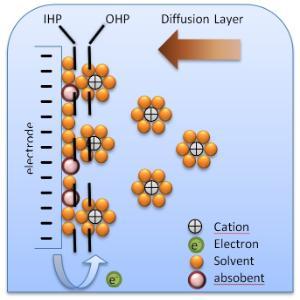
Figure 1. Schematic electrical double layer (2)
Electrical double layer consist of:
- The Inner Helmholtz Plane (IHP) is an ionic layer that consists of adsorbed dipole molecules. Only specific adsorbed anions bond to metal surface.
- The Outer Helmholtz Plane (OHP) consists of a plane of adsorbed solvated cations.
- The Diffusion Layer (DL) is a thick layer located in a region of diffuse ions in contact with the OHP and the bulk of solution.
Adsorption of organic inhibitors on metal surface:
Once the organic molecule reaches the metal surface, adsorption of the inhibitor molecule takes place. This will led to a change in the potential difference between metal surface and electrolyte as a result of unbalanced electric charge distribution at metal-solution interface (3).Adsorption of organic molecule on metal surface is governed by residual charge on the surface of the metal and chemical structure of inhibitor (3). The two main types of the adsorption of an organic inhibitor on a metal surface are physical and chemisorption. Chemisorption is the transfer, or sharing of the inhibitor molecules charge to the metal surface, forming a coordinate-type bond. Chemisorption is probably the most important type of interaction between the metal surface and inhibitor molecule (3).an inhibitor molecule
Functional atoms:
An organic molecule should have reactive atoms which are the active site of chemisorption process and the strength of chemisorption relies on the electron density and polarizability of these functional atoms (3). The effectiveness of functional atoms in the adsorption process varies according to the following sequence:
Selenium > Sulfur > Nitrogen > Oxygen
This order is explained by lower electronegativity of elements on the left that make their compounds easily susceptible to polarization (4).
Hard and soft base Principle:
In the view of hard and soft acid/base theory that was propounded by Ralph G.Pearson (5), which states that hard acids prefer to bind to hard bases and soft acids prefer to bind to soft bases. This rule was applied in studying corrosion inhibition mechanisms. Organic inhibitors can be classified as soft and hard inhibitors based on their polarizability(12), soft molecules possess high polarizability and hard molecules possess low polarizability. Neutral metal atoms are soft acids, so they prefer to bond soft bases like Sulphur-containing inhibitors. In turn, Nitrogen-containing inhibitors are considered to be hard bases. Therefore, they interact with neutral metal atom less strongly than sulfur containing inhibitors (3),(6). It should be noted that iron(II) would be considered much softer than Al(III). Thus an inhibitor which is good for iron would not generally be good for aluminum.
Chemical structure effect:
Many studies revealed that an increase in the electronic charge on the functional atom results in promoting the ability of organic molecule to be adsorbed to metal surface, thus enhance inhibition efficiency. Hackerman and his co-workers (7) establish that the inhibition efficiency enhanced by increasing electron density at function atom. More investigations conducted by Trabanelli (8) backed this theory when studying alicyclic amine compounds, concluding that the increase in inhibitors effectiveness was related to the high electron density around nitrogen atom.
Early studies indicated that organic compounds of asymmetrical structure are more effective inhibitors than compounds of symmetrical structure(4). Foster and co-workers(11) study on acetylene inhibitors concluded that symmetrical compounds have poor inhibitive properties. Not many studies support this statement.
The availability of π electrons due to the presence of multiple bonds or aromatic rings would facilitate electron transfer from inhibitor to the metal besides enhancing the electron density on the donor atom. This effect can also be influence by introducing electron donating and withdrawing substituents in suitable position in molecule(3).
Hammett equation:
According to Hammett (9), the ratio of reaction rate constant k1 for organic compound with a substituent to the reaction rate constant k2 of the organic compound without a substituent is given by the following equation:
log (k1/k2)= ρ σ
Where; ρ is constant for given reaction and σ reflects the influence of substituent on the electron density of reaction center of molecule
The Hammet equation has been applied in corrosion inhibitor studies. The effectiveness of inhibitors can be correlated with Hammett’s σ parameter. Sastri and Perumareddi (10) conclude that the corrosion rate for pyridine derivatives as a function of σ values obtained from Hammet equation decreased or the percent inhibition increased as the negative σ value increased. The negative σ value indicated the electron donating property of the substituent.
Conclusion:
To be considered as an effective inhibitor, organic molecule should meet the following requirement.
- Has electron donor atom, Sulphur or Nitrogen.
- Has Substituent that increase electron density around electron donor atom.
- The presence of aromatic, heterocyclic ring or multiple bonds is enhancing protective properties.
- Inhibitor should be effective at low concentration.
References:
- Nestor. Perez, “Electrochemistry and Corrosion Science”, Kluwer Academic Publishers,2004.
- Byoung-Yong Chang and Su-Moon Park, 2010, Annu. Rev. Anal. Chem. 2010. 3:207–29
- Sastri, V. S., "Corrosion inhibitors Principle and Applications", John Wily & Sons. Inc., 1998.
- I.L.Rozenfeld.,Corrosion Inhibitors, McGraw-Hill Inc ,1981
- R. G. Pearson, Journal of Chemical Education, 1968,V.45(9), p 581-587.
- Aramaki. K, Mochizuki T, Nishihara H, 1987 Proc.10th Int. Congress on Metallic Corrosion, Vol.3, p 2759.
- R.R.Annand ; R. M. Hurd , and N. Hackerman(1965) J.Electrochem.Soc. Vol. 112, No. 2, p138-144.
- G.Trabanelli, V.Carassiti, , Advanced in Corrosion Science and Technology, Plenum Press,1970, Vol.1
- Hammett,L.P., Chem.Rev., 1935,17(1),p125-136
- Sastri and Perumareddi, Corrosion,1994,Vol. 50, No. 6,p432-437
- G.L. Foster, B.D. Oakes, C.H. Kucera, Industrial and Engineering. Chem. (1959), Vol.51 (7) p825.
- V.S. Sastri, P.R. Roberge, Proc. 11th Int. Corrosion Congress,1990, Florence, Italy,Vol.3, p 55.
