What’s Happening at Missouri S&T:
(formerly UMR)
Introduction to Paint Formulation
There is still time to register for the Fall 2008 "Introduction to Paint Formulation" course November 3-7, 2008! This year's course should be very timely since the cost of raw materials is escalating and any savings is more important than ever.
The "Introduction to Paint Formulation" course approaches formulation from a PVC point of view relating scientific principles to the paint’s formulation and performance. This is a lecture and lab oriented course. The lab portion has been modified to include water borne alkyds and UV cure systems. The classic latex, water reducible bake, polyester bake, water borne urethane, water borne epoxy, and solvent borne alkyd coatings are also all still available for the laboratory portion of the class. Monday will be all lecture with half day lecture half day lab the rest of the week. Tuesday evening we will have 5 graduate students and myself help the class formulate the coatings they will make in lab the rest of the week, starting from the resin product literature. The formulation includes the calculations of PVC, CPVC, and other important parameters, as well as choosing the raw materials that will go into the coating.
The course has proven to be extremely helpful to new and experienced formulators, raw material sales, marketing, QC batch adjusters, OEM specifiers, paint technicians and anyone else who could benefit from learning this material. The cost of this course is $1145.
To register send an e-mail to:Michael R. Van De Mark at mvandema@mst.edu and include the participants name, mailing address, e-mail address, phone number, fax number and method of payment. We will send information back in the form of an e-mail.
We hope to see you or a colleague of yours in November!
Michael R. Van De Mark, Director
Missouri S&T Coatings Institute
Technical Insights on Coatings Science
What Controls 2K Curing Speed?
By Robert Hull, Graduate Student, Missouri S&T Coatings Institute
Most coatings are single package systems with the “binder,” the polymer material that forms the actual film of the coating, integral with the pigment dispersion. These types of systems are known as 1K and are typical of most architectural coatings. However, coating systems which require very high molecular weight, cross-linked polymer binders often have to be prepared just before or during application by the chemical reaction of two components. These two-component or “two-package” (2K) systems are common with urethane and epoxy coatings and typically achieve higher strength and durability, though one of the primary drawbacks to these systems is that the two chemicals must be kept separate until ready for application. For urethanes these coatings are formed by the reaction of an isocyanate component with an alcohol component where the alcohol component is usually some chemical species that contains multiple alcohol groups per molecule, which allows for cross-linking.
Once the components of a two-package system are mixed a chemical reaction is initiated at the end of which a solid, cross-linked polymer film will be formed. As the reaction proceeds, the molecular weight and cross-link density increase which in turn increases viscosity of the system. Thus, there is a limited amount of time after initiation of the reaction to apply the coating before the viscosity becomes too high. This is called the “pot life” of the system. While it is usually desirable to maximize the pot life, it must be balanced against the “cure-time” of the coating, the amount of time before it forms a film of sufficient strength for its application, as these factors are essentially in opposition: a longer pot life also means a longer cure time, which is almost always undesirable. Optimization of the system usually involves a compromise between the two. To control these aspects when designing a coating it’s necessary to consider the basic parameters affecting the rate of the reaction as this dictates both the pot-life and cure-time.
The rate of a chemical reaction is basically the rate at which the reacting species collide with enough energy to form a product:
A + B ------> A-B
This can be written as the concentration of species A times the concentration of species B times the rate constant, k,
Rate = k[A][B]
where the rate constant is
k = Ae-Ea/RT
where T is the temperature, R is the universal gas constant, the pre-exponential factor A is essentially the rate at which collisions occur and the term e-Ea/RT is the fraction of collisions occurring with energy greater than the minimum, Ea, (the activation energy) required for the two to form the product. Thus, it’s readily apparent that the reaction rate, and thus the pot life and cure time, is implicitly dependent on the concentration of the reactive species (the reacting isocyanate and alcohol groups), the temperature of the system and the minimum energy required to form a product after collision.
From the above relationships it is seen that increasing the temperature will increase the rate constant and thus the reaction rate. Elevated temperatures will then decrease both the pot life and the cure time and an optimal temperature for the two must normally be achieved. A general rule of thumb is that for every ten degrees Centigrade the temperature is increased, the reaction rate is doubled. Conversely, for every ten degree decrease the reaction rate is halved.
The activation energy, Ea, can be adjusted by using a catalyst, a chemical compound which has the net effect of lowering the apparent activation energy. This in turn increases the rate constant, k, and thus increases the rate. An illustration of this effect is shown below.
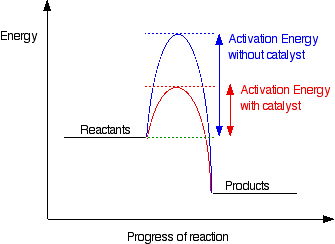 |
http://www.chemguide.co/uk/physical/basicrates/catalyst.html
For example, reactions of isocynates and alcohols can be catalyzed by diazabicyclo[2.2.2]octane (DABCO for short) or dibutyl dilaurate (DBTDL). Or the two can be utilized together synergistically. Typically a variety of different catalysts are available and changing the identity of the catalyst can have dramatic effects on the reaction rate.
And finally, though it’s not obvious from the general rate equation given above, changing the solvent will also have a significant effect on the reaction rate. A “faster” solvent, one that evaporates quickly, will increase the reaction rate as it will increase the concentration of the reactants quicker. Similarly, a slower solvent will act to decrease the reaction rate. Changing the solvent, however, can create other complications as coating systems are generally very sensitive to even slight variations in solvent properties.
In sum, the kinetics of a two-component coating system can be controlled by the temperature and the solvent and catalyst used. Keeping these three parameters in mind when designing a coating system can give ample leeway in the range of pot life and cure time. The new high solids coatings take advantage of lower molecular weight polyol and isocyanate. Thus the concentration of alcohol and isocyanate groups is higher than in conventional urethanes. The effect of this is to decrease pot life, however since the number of bonds needed to for the final high molecular weight polymer is now much higher, the cure time of the film increases. The shorter pot life and extended cure time are a major problem for OEM and maintenance workers. Thus potentially lowering pot temperature and increasing the temperature during cure will solve the problem in most cases, consistent with the above.
