Volume 2 Issue 9
What’s Happening at Missouri S&T:
Spring Short Courses
This spring we will be offering “Basic Composition of Coatings¿? March 13-17, 2006 and “Introduction to Paint Formulation¿? May 15-19, 2006. The Basic Composition course is intended for new personnel in the coatings profession. It targets the components of coatings (resin, pigments, extenders, solvents and additives), testing and specifications, general formulation and manufacturing methods. Basic Composition is primarily a lecture course with several laboratory demonstrations. The Introduction to Formulation course is intended to give the person a fundamental knowledge of how to approach a starting formulation and troubleshoot it. This course involves both lecture and laboratory work For more information see our web site at http://coatings.mst.edu and to register contact Michael Van De Mark at coatings@mst.edu or call 573-341-4419.
Summer Interns
Remember to contact us early if you are planning to hire a summer intern next year. We will make sure that our students are informed of the opportunity.
Technical Insights on Coatings Science
Adhesion to Plastics: A New Look at How to Achieve Adhesion
By Thomas Schuman
Adhesion to plastic materials is a critical but troublesome property to attain for coatings applied to molded plastic objects, such as automobile bumpers or toys. The drive to use plastics includes advantages especially in the areas of mass density (weight savings), toughness, and/or cost. Appearance and its durability is a matter of consumer expectations, safety, and design parameters. Adhesion is critical not only for durability but also because adhesion heavily influences the physical properties of the coating. The coating and substrate together constitute a two-dimensional composite structure, a teamwork approach, to attain a desired set of physical properties, such as hardness, scratch resistance, chip resistance, and gloss that are affected by the underlying surface’s hardness, smoothness, and adhesion to the coating.
Adhesion = Surface area * (Bond strength/unit area) [1]
The magnitude of adhesion is proportional to the amount of interfacial contact area between the coating and substrate and a bond strength per unit area. Therefore, good wetting gives optimal surface contact and adhesion but wetting alone does not provide good adhesion if the bond strength is low. Rough surfaces create more surface area but are more difficult to wet, i.e., rough surfaces require a lower contact angles to ensure efficient surface wetting.
Seemingly simple, achieving adhesion to plastics is not easily obtained since most plastics generally have low surface tension, are difficult to wet, and they produce low bond strengths to surface coatings. Interpolymer mixing of different polymer types across the substrate-coating interface does not usually occur. Manufacturing (molding) conditions or surface treatments to improve adhesion at the surface can cause the formation of a weak boundary layer, a subsurface region of poor cohesion which will fail causing delamination. Plastics are typically molded against surfaces that are quite polished, and the smooth polymer surfaces make interfacial contact area problematic but are necessary to keep the polymer from sticking to the mold surface. Herein lies the solution to plastic adhesion.
Methods utilized to improve adhesion to molded polyolefins, which are waxy and particularly difficult to coat, are generally thought to increase bond strengths by increasing surface polarity – they generate polar groups on the surface that are wettable and stick better. Common methods include flaming, corona, or air plasmas, an oxidizing etch (like acidic permanganate), a free radical initiator, surface swelling by solvents, or adhesion promoting compounds (such as chlorinated polyolefins). Each process has advantages and disadvantages: Flaming, corona, and air plasmas suffer in their ability to treat complex part surfaces that can result in some areas with little to no treatment and other surface regions with over-treatment. Chemical etching or free radical processes are expensive and hazardous to personnel. Solvent soaking appears inconvenient and is not understood in terms of how to choose an appropriate solvent. Although chlorinated polyolefin adhesion promoters (ad-pros) are relatively inexpensive and serve as a convenient means of improving adhesion to low energy surfaces such as polyolefins, it is also known that the solvent or cosolvent used in the ad-pro formulation is critical to optimize performance.
Recent work has shown that surface treatments that are effective alter the surface topographical profile. Solvents may or may not produce a change in the topographical profile of the plastic surface, but in two recent examples only solvents that induced a change in topography caused improvements in adhesion.1 The amount of surface swelling by the solvent, or other coating formulation considerations, such as solubility parameter matching of the solvent to the polymers, had little bearing on whether adhesion resulted. In the case of polypropylene, a very fine grained surface topography resulted upon exposure to tetrahydrofuran (see Figure 1); and, tetrahydrofuran in a waterborne coating improved coating adhesion to polypropylene (see Figure 2). For ethylene-styrene interpolymers, aromatic 100 solvent (such as Cyclosol® 100) caused a much larger change in the surface topography compared to toluene or xylene; aromatic 100 added to the solventborne lacquer formulation increased coating adhesion. It was concluded that solvents affect coating adhesion to a plastic substrate by altering the surface topographical profile of the plastic substrate. The changes in topographical profile would seem to result from differential swelling of localized polymer compositions by the solvent or a solvent-induced relaxation of the localized domains. De-swelling the surface through solvent evaporation leaves an altered profile compared to the untreated substrate.
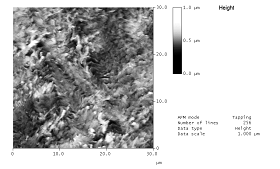 |
| a) b) Figure 1. Atomic force microscopy (AFM) images (30x30?m) of a polypropylene surface a) before, as received; and b) after exposure to tetrahydrofuran. Brightness is directly proportional to surface height from 0 (black) to 1?m (white) (from reference 1). |
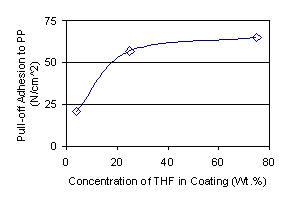 |
| Figure 2. Coating adhesion to polypropylene as a function of solvent concentration (from reference 1). |
Other support for this hypothesis shows that a corona process, commonly used for improving adhesion, similarly induces changes in the topographical profile of various treated plastic substrates, e.g., causing the formation of nodules2-4 in addition to changes in the surface polarity. Two other articles describe the effects of topography on adhesion.5,6 The act of grit blasting of surfaces to improve adhesion takes on new meaning as to the effect of a profile on coating adhesion in light of these results. Moreover, another recent article demonstrated the effect of surface profile on the magnitude of adhesion based on creating model surfaces to mimic those of natural adherent or abherent surfaces.7 The model surfaces were created by adding cylinders that varied in topographical height, radius, and placement, i.e., their number and separation, on a surface. The magnitude of adhesion was found to be altered according to the height and placement of the surface features.
Surface topography plays a direct role in the magnitude of surface adhesion. Surface profiling by, e.g., grit blasting of metals, similarly takes on new meaning as to specifying the need for a particular profile geometry. The role of solvents in the coating of plastic substrates is to alter the surface topographical profile, where the size and placement of the surface features affects the coating adhesion.
Further Reading
1 T.P. Schuman, S.F. Thames, “Coating solvent effects producing adhesion to molded plastic parts,¿? Journal of Adhesion Science and Technology, 19, 1207-1235 (2005).
2 V. Jones, M. Strobel, M. J. Prokosch, “Development of polypropylene surface topography during corona treatment,¿? J. Plasma Processes and Polymers, 2(7), 547-553 (2005).
3 C. Weiss, H. Muenstedt, “Surface modification of polyether ether ketone (PEEK) films for flexible printed circuit boards,¿? Journal of Adhesion, 78(6), 507-519 (2002).
4 G. J. Vancso, T. D. Allston, I. Chun, L. S. Johansson, G. Liu, P. F. Smith, “Surface morphology of polymer films imaged by atomic force microscopy,¿? International Journal of Polymer Analysis and Characterization, 3(1), 89-105 (1996).
5 D. E. Packham, “Surface energy, surface topography and adhesion,¿? International Journal of Adhesion and Adhesives, 23(6), 437-448 (2003).
6 L. T. Drzal, N. Sugiura, D. Hook, “The role of chemical bonding and surface topography in adhesion between carbon fibers and epoxy matrixes,¿? Composite Interfaces, 4(5), 337 – 354 (1997).
7 A. J. Crosby, M. Hageman, A. Duncan, “Controlling polymer adhesion with “Pancakes¿?,¿? Langmuir, 21, 11738 – 11743 (2005).
The End User
How is Profile Measured?
By Michael Van De Mark, Director, Missouri S&T Coatings Institute
As shown in the article above, atomic force microscopy (AFM) can be used to image small areas of a panel. Atomic force microscopy is an excellent research tool and gives details on an atomic scale. Other methods usually use a simple probe method ASTM D4417 Method B. Ultrasonic microscopy, scanning electron microscopy and conductivity probes can also be utilized. Cost and speed of measurement are critical for most applications and thus limit the resolution. Surface preparation method development can utilize the more costly and slower technologies to gain detailed information. In the field or on the line, speed and a simple pass or fail answer is needed. Digital surface roughness probe systems typically measure 0.05-50 micron surface profile with cost between $1,400 and $5,000.
 |
A low cost surface profile gage for sand, shot or grit blast surface measurements.
Is there a topic you would like discussed? Contact us by e-mail at coatings@mst.edu.
| March 13-17, 2006 Basic Composition of Coatings This course provides an overview of the components of paint and how they work. Participants are also introduced to methods for testing and manufacture of paint. |
| May 15-19, 2006 Introduction to Paint Formulation This course provides techniques used in formulating paint from raw materials. It involves formulating and making paint in the laboratory, "Hands on!" |
To subscribe/unsubscribe to this newsletter, click here. Feel free to forward to this your colleagues. |
