VOLUME 16 ISSUE 4
WHAT’S HAPPENING AT MISSOURI S&T (FORMERLY UMR):
SHORT COURSE DATES
We will be offering "Introduction to Paint Formulation" May 18-22 (Spring 2020). This course is intended to give the person a fundamental knowledge of how to approach a starting formulation and troubleshoot it. This course involves both lecture and laboratory work.
For more information see our web site at http://coatings.mst.edu and to register contact us at mstformulation@mst.edu or call 573-341-4419. **These courses are held on the Rolla Campus**
ONLINE SHORT COURSE
We are offering "Introduction to the Coating Systems" online short course. This course is targeted for automotive and aviation type OEM companies. This self-paced seminar will cover the painting system from the composition of paints to the evaluation of the dry film. The pigments, resin, solvents and additives will be discussed including their influence on the coatings performance. Color measurement, surface profile, and other evaluation criteria will be related to composition. The importance of surface preparation and other manufacturing criteria will show the system complexity and each step's importance.
We are offering "Surface Defects: Elimination from Human and Process Contaminants" online short course. This course addresses many of the issues in prevention and minimization of defects. The course covers the defects caused by the coatings process, as well as human issues, including personal care product causes. Several of the surface defects are discussed – from basic principles and real world automotive and aircraft examples. The highly practical approach of this course will greatly aid the personnel involved in the painting operation to reduce and systematically approach issues.
EMPLOYMENT TAB
Anyone wanting to have job opening listed, please contact us at (573) 341-4419 or e-mail: mstformulation@mst.edu . You can also write to us at Missouri S&T Coatings Institute, BOM #2, 651 W. 13th St., Rolla, MO 65409-1020. Our web site is http://coatings.mst.edu
TECHNICAL INSIGHTS ON COATINGS SCIENCE
Anti-freeze for coatings
Peng Geng, Research Assistant, Missouri S&T Coating Institute, Missouri University of Science and Technology
Water-borne coatings use water as the dispersion medium and diluent. Compared with traditional organic solvent-borne coatings, water-based coatings save a lot of organic resources which greatly reduces the atmospheric pollution caused by the volatilization of organic solvents in coatings.1 Therefore, water-borne coatings have become the mainstream of the coating industry. However, during transportation and storage, the large amount of water in coatings will freeze at low temperature, and cause coating defects.2 Thus, in order to achieve better free thaw stability, addition of anti-freeze is necessary. Antifreeze is an additive that can prevent part of the water from freezing at low temperature by lowering the freezing point. Thus providing a layer of liquid around latex particles that keep the particles separated. The freeze thaw resistance can be evaluated by separating a water-borne coating into two pint-size (500mL) resin-lined cans, keeping one can at room temperature, while other can is going through cycles of freezing and thawing processes. After cycling, the coating in both cans are examined for any change of viscosity and visual film properties.3
Various anti-freeze agents have been developed and utilized over the past few decades. The most common anti-freezes are glycols, such as ethylene glycol and propylene glycol.4 Ethylene glycol can be converted by the liver into four chemicals that are toxic. Propylene glycol is more expensive but considered non-toxic compared with ethylene glycol. Both ethylene glycol and propylene glycol are very efficient and allow coatings to be still useful even after five freeze thaw cycles.5,6
When water-based coatings are exposed to cold temperature conditions, water will start to freeze and form ice crystals. As the crystals grow, there is less and less liquid water existing in the dispersion and therefore the concentration of latex particles increases in the remaining liquid, and the latex particles are forced close together. This increases the potential particle aggregation which result in undesirable coagulation and coalescing of latex particles,7 shown in Figure 1.
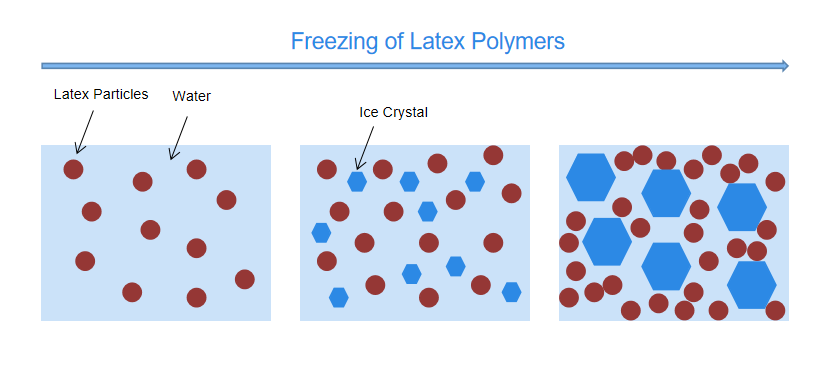
Figure 1. Freezing of Latex polymers
For a low Tg polymer, the chains can still flow and particles coalescence at lower temperature, therefore the coagulation and coalescing problem is more serious. Because water/glycol mixtures have a much lower freezing point, the use of glycol can inhibit part of water from freezing which keep a layer of non-freezing liquid around each latex particle, stabilizing the coating. However, many disadvantages have limited the usage of glycols. Both ethylene glycol and propylene glycol are volatile. In many situations, in order to maintain a low level VOC the freeze thaw ability has to be compromised. In addition, without the presence of inhibitor, glycols can react with oxygen and metal ions, thus forming organic acids and accelerating the corrosion of metals in the system.5,6
Colloidal Unimolecular Polymer (CUP) particles are a new class of nanoscale material. It is a potential candidate to replace the traditional glycols as anti-freeze or the co-resin for latex and polyurethane dispersions. The polymer can be simply synthesized and made into colloidal form by a water reduction process. CUP is spheroidal and has a particle size ranging from 3 to 9 nm. Also, during the water reduction process, all the volatile organic contents were completely removed, CUP has zero VOC.8 These particles generally associate with a large amount of non-freezable surface or bounded water (about the same weight percent as CUP solids), due to the hydrophilic groups on the surface,9 shown in Figure 2.
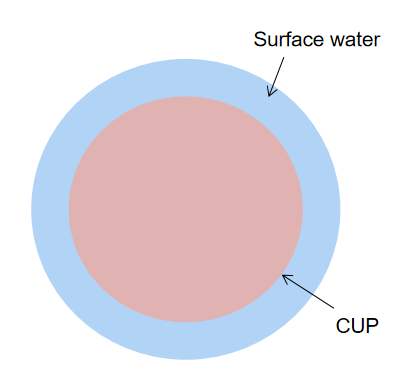
Figure 2. CUP with surface water around the solid
According to the previous study by Van De Mark et.al., the amount of surface water increases with the decreasing of particle size assuming the surface water thickness is constant. This increased water is because at the same solid percent, smaller particles have larger surface area.10 Using a latex formulation as an example, it contains 30% (by volume) latex particles, 20% pigment particles and 50% water. If we replace 1/3 latex particles by CUP particles as a co-resin (5 nm particle size as an example), there are approximately 10% surface water, 20% latex particles, 20% pigment particles and 40% ice when all water freezes. Therefore, the number of particles of CUP to latex is 4000:1, and the total surface area of CUP particles is 10 times larger than latex particles. Thus, when all the free water freezes, CUP can maintain sufficient non-frozen fluid that prevent the latex particles from contacting with each other, thus increasing the freeze thaw stability, shown in Figure 3.
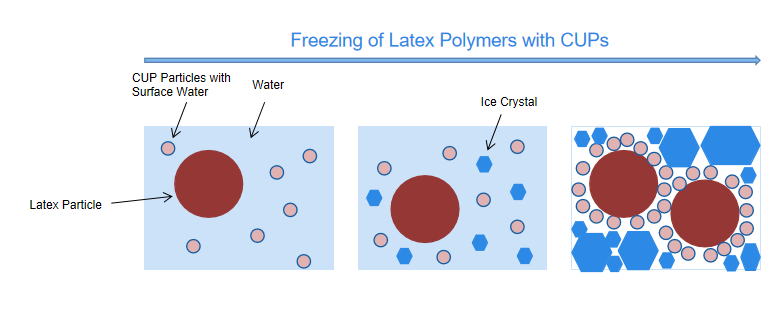
Figure 3. Freezing of Latex Polymers with CUPs
Reference
- Wolkoff, P., Wilkins, C.K., Clausen, P.A., Nielsen, G.D., "Organic compounds in office environments - sensory irritation, odor, measurements and the role of reactive chemistry," Indoor Air. 16, 7-19 (2006)
- Natu, A., Van De Mark, M.R., “Synthesis and Characterization of an Acid Catalyst for Acrylic-melamine Resin System based on Colloidal Unimolecular Polymer(CUP) Particles of MMA-AMPS,” Progress in Organic Coatings, 81, 35-46 (2015)
- ASTM D2243-95, Standard Test Method for Freeze–Thaw Resistance of Water-Borne Coatings, 2008.
- Bosen, S.F., Bowles, W.A., Ford, E.A., Person, B.D., “Antifreezes”, Ullmann’s Encyclopedia of Industrial Chemistry, A3, 5th ed. (1985)
- PM Leth, M Gregersen. Ethylene glycol poisoning. Forensic science international, 2005 - Elsevier
- Evaluation of Certain Food Additives and Contaminants (Technical Report Series). World Health Organization. 105. ISBN 92-4-120909-7.
- Gade, S.V., “Application of Colloidal Unimolecular Polymer(CUP) Particles in Coatings,” Doctoral Dissertation, 2446 (2015)
- Riddles, C., Zhao, W., Hu, H.J., Chen, M., Van De Mark, M.R., “Self-assembly of Water insoluble Polymers into Colloidal Unimolecular Polymer (CUP) Particles of 3-9 nm,” Polymer, 55, 48-57 (2013)
- Alabarse, F.G., Haines, J., Cambon, O., Levelut, C., Bourgogne, D., Haidoux, A., Granier, D., Coasne, B., “Freezing of Water Confined at the Nanoscale,” Phys. Rev. Lett., 109, 035701 (2012)
- Chen, M., Riddles, C.J., Van De Mark, M.R., “Electroviscous Contribution to the Rheology of Colloidal Unimolecular Polymer(CUP) Particles in Water,” Langmuir, 29(46), 14034-14043 (2013)
