VOLUME 15 ISSUE 6
WHAT’S HAPPENING AT MISSOURI S&T (FORMERLY UMR):
SHORT COURSE DATES
We will be offering "Introduction to Paint Formulation" Oct 21-25 (Fall 2019). This course is intended to give the person a fundamental knowledge of how to approach a starting formulation and troubleshoot it. This course involves both lecture and laboratory work.
For more information see our web site at http://coatings.mst.edu and to register contact us at mstformulation@mst.edu or call 573-341-4419. **These courses are held on the Rolla Campus**
ONLINE SHORT COURSE
We are offering "Introduction to the Coating Systems" online short course. This course is targeted for automotive and aviation type OEM companies. This self-paced seminar will cover the painting system from the composition of paints to the evaluation of the dry film. The pigments, resin, solvents and additives will be discussed including their influence on the coatings performance. Color measurement, surface profile, and other evaluation criteria will be related to composition. The importance of surface preparation and other manufacturing criteria will show the system complexity and each step's importance.
We are offering "Surface Defects: Elimination from Human and Process Contaminants" online short course. This course addresses many of the issues in prevention and minimization of defects. The course covers the defects caused by the coatings process, as well as human issues, including personal care product causes. Several of the surface defects are discussed – from basic principles and real world automotive and aircraft examples. The highly practical approach of this course will greatly aid the personnel involved in the painting operation to reduce and systematically approach issues.
EMPLOYMENT TAB
Anyone wanting to have job opening listed, please contact us at (573) 341-4419 or e-mail: mstformulation@mst.edu . You can also write to us at Missouri S&T Coatings Institute, BOM #2, 651 W. 13th St., Rolla, MO 65409-1020. Our web site is http://coatings.mst.edu
TECHNICAL INSIGHTS ON COATINGS SCIENCE
Surfactant
Peng Geng, Graduate Research Assistant, Missouri S&T Coating Institute
Surfactants are a group of compounds that can significantly change the interface state of a solution system at low concentration. Surfactant is an amphiphilic structure that has a fixed number of hydrophilic and lipophilic groups that can be aligned on the surface of the solution.1 The molecular structure of the surfactant is amphiphilic: one end is a hydrophilic group and the other end is a hydrophobic group. The hydrophilic group is a polar group such as carboxylic acid, sulfonic acid, sulfuric acid, amino group or amine group and their salts.2 Also, hydroxyl group, amide group and ether are considered as polar hydrophilic groups. The hydrophobic group often includes a non-polar hydrocarbon chain with eight or more carbon atoms, shown in Figure 1. Surfactants are classified into ionic surfactants, nonionic surfactants, amphoteric surfactant, and complex surfactants.
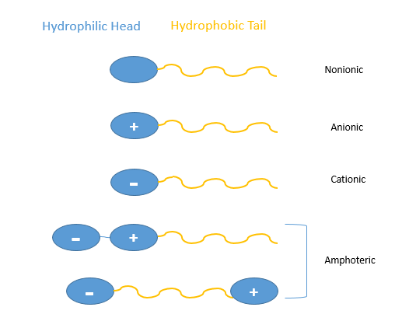
Figure 1. Types of surfactant
The surfactant, when dissolved in water, is classified as an ionic surfactant or a nonionic surfactant based on whether the ions are generated or not. Most commonly, surfactants are classified according to the polar head groups: a nonionic surfactant has no charge on the end; the ionic surfactant has positive or negative charges on the end; if a surfactant contains both positive and negative charges, then it’s called zwitterionic.3 Furthermore, ionic surfactant can be defined as cationic and anionic depending on the charge is positive or negative at the end of the hydrophobic chain. Anionic surfactant contains anionic functional groups like sulfate, sulfonate, phosphate and carboxylates. Carboxylate surfactant sometimes will form a precipitate in the presents of calcium and magnesium salts. Sulfonates and sulfates are detergents and tolerate hard water.4 Cationic surfactant is usually pH-independent, it can exhibit high water solubility and are stable in acidic and alkaline solutions, also it has good surface activity and anti-bacteria activity.5 When a cationic surfactant is added to a paint it will usually cause flocculation or precipitation due to charge charge attraction since most pigments at the slightly alkaline pH of paint have a negative charge on their surface. Zwitterionic surfactant has both positive and negative charges in the structure and can exhibit cationic and anionic surfactant properties in different pH conditions. The cationic part is mainly primary, secondary or tertiary amine salts. The anionic part is more variable, often includes sulfonates. The nonionic surfactant means non-ionizing in an aqueous solution, and its hydrophilic group is mainly composed of a certain number of oxygen-containing groups, usually based on short polyethylene oxide chains. The lack of ionic repulsion is the reason that the nonionic surfactant is superior to the ionic surfactant in some aspects.6 It is not in the ionic state in solution, it is more stable and not easily affected by the presence of strong electrolyte inorganic salts. The non-ionic surfactants are also pH-independent and compatible with other type of surfactants. When surfactants are added, as the interface is created, the adsorption is controlled by the diffusion coefficient of the surfactant to the interface. In some cases, if an energetic barrier exists to limit the rate for adsorption or desorption, the dynamic is then called kinetically limited. These energy barriers are usually due to structure steric or electrostatic repulsion. The surface rheology of the surfactant is important in stabilizing foams and emulsions.
When the surfactant in water is at a low concentration, it is in a molecular state and dispersed in water. Surfactants reduce the surface tension of water by adsorption at the interface between air and water or at the interface between oil and water. When the concentration is gradually increased to a certain point, many surfactant molecules can aggregate into a large group to form aggregates (micelles) in bulk solutions. This point is called critical micelle concentration (CMC), it’s defined as the minimum concentration required to form micelles.7 An aggregation number is used to describe the number of surfactant molecules that are associated with the micelle, it’s usually measured by light scattering.
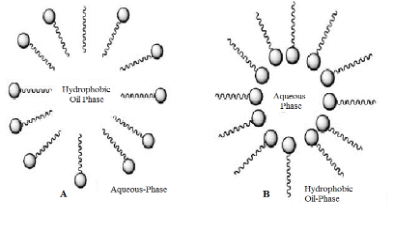
When a surfactant is dissolved in a non-polar organic solvent, and the concentration exceeds the critical micelle concentration, the micelle formed in the organic solvent is called reverse micelle, in which the non-polar groups are externally contacted with a non-polar organic solvent, while the polar groups are aligned to form a polar core. This polar core has the ability to dissolve polar substances, and after the polar core dissolves in water, it forms a “water pool”, shown in Figure 2. Reverse micelles are a spontaneously formed nanoscale aggregate that is a transparent, thermodynamically stable system.8
Figure 2. A. micelle; B. reverse micelle
In addition, depending on the desired properties and the particular application, surfactants are sometimes required to have different hydrophilic and lipophilic structures and relative densities. By changing the type of hydrophilic or lipophilic group, percentage and its position in the molecular structure, different hydrophilic-lipophilic balance HLB values can be achieved. Hydrophilic-lipophilic balance number (HLB) is a measurement of the equilibrium relationship between hydrophilic groups and lipophilic groups in a surfactant molecule, it is a relative value and generally limited to 0-20 for nonionic surfactant. It was first proposed by Griffin in 1949 for nonionic surfactant,9, 10 and it’s described as the equation below:
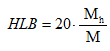
Mh: molecular mass of hydrophilic portion of the surfactant molecule
M: molecular mass of the whole molecule
Based on the Griffin’s method, the HLB value was set to be 0-20, the saturated alkane group with the largest hydrophobicity was set to 0, while the surfactants consisting entirely of hydrophilic polyoxyethylene has an HLB value of 20. The larger HLB value, the stronger the hydrophilicity.
Surfactants are a flexible, practical chemical product with a wide range of application, such as lower surface tension, detergents for extracting proteins, also as wetting agents, dispersants, emulsifier and etc. For example, wetting agent is a substance that can dissolve in water, lower the contact angle, replacing an air phase to a liquid phase at the surface, and wet a hydrophobic pigment that is insoluble or not wetted by water, which is very important for paint. A proper wetting agent can also ensure the coating to wet to a more uniform surface, including low surface energy substrates, defective and dirty surface.11 Cationic surfactant will cause precipitation in latex paint, so only nonionic and anionic can be used. Emulsifier is used to improve the surface tension between various constituent phases in the emulsion to form a uniform and stable dispersion or emulsion.12 The emulsifier is a surface active substance having both a hydrophilic group and a lipophilic group in the molecule, which aggregates at oil/water interface, can reduce the interfacial tension and reduce the energy required to form the emulsion, thereby increasing the stability of the emulsion. The latex resin is synthesized by adding an emulsifying surfactant to water, which forms micelle, followed by the monomer and an inhibitor. The monomers are stabilized inside the micelle and as it is polymerized the micelle grows due to the addition of more monomer. The number of particles is controlled by the amount of surfactant and the diameter of the latex particles is controlled by the amount of monomer added.13 Detergent is a surfactant that specially formulated for cleaning purpose. The main components are usually composed of alkylbenzenesulfonates, which is similar to soap but more soluble in hard water.14 They are mostly amphiphilic, the hydrophilic and hydrophobic dual nature can facilitate the hydrophobic compounds with water.
Reference:
- 1. Rosen MJ & Kunjappu JT (2012). Surfactants and Interfacial Phenomena (4th ed.). Hoboken, New Jersey: John Wiley & Sons. p. 1. ISBN 978-1-118-22902-6. Archived from the original on 8 January 2017.
- 2. "Bubbles, Bubbles, Everywhere, But Not a Drop to Drink". The Lipid Chronicles. 11 November 2011. Archived from the original on 26 April 2012. Retrieved 1 August 2012.
- 3. IUPAC Gold Book zwitterionic compounds/zwitterions
- 4. Colloid and surface chemistry, Biermann’s Handbook of Pulp and Paper, v2, 2018, p 381-400
- 5. "Bordwell pKa Table (Acidity in DMSO)". Retrieved 11 May 2014.
- 6. Kurt Kosswig "Surfactants" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, 2005, Weinheim. doi:10.1002/14356007.a25_747
- 7. IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "critical micelle concentration". doi:10.1351/goldbook.C01395
- 8. Hakiki, F., Maharsi, D.A. and Marhaendrajana, T. (2016).Surfactant-Polymer Coreflood Simulation and Uncertainty Analysis Derived from Laboratory Study. Journal of Engineering and Technological Sciences. 47(6):706-724. doi: 10.5614/j.eng.technol.sci.2015.47.6.9
- 9. Griffin, William C. (1949), "Classification of Surface-Active Agents by 'HLB'" (PDF), Journal of the Society of Cosmetic Chemists, 1 (5): 311–26
- 10. Griffin, William C. (1954), "Calculation of HLB Values of Non-Ionic Surfactants" (PDF), Journal of the Society of Cosmetic Chemists, 5 (4): 249–56
- 11. Hakiki, F.; Maharsi, D.A.; Marhaendrajana, T. (2016). “Surfactant-Polymer Corefllod Simulation and Uncertainty Analysis Derived from Laboratory Study”. Journal of Engineering and Technological Sciences. 47(6): 706-724. doi:10.5614/j.eng.technol.sci.2015.47.6.9.
- 12. Ozhovan M.I. (1993). "Dynamic uniform fractals in emulsions"(PDF). J. Exp. Theor. Phys. 77 (6): 939–943. Bibcode:1993JETP...77..939O.
- 13. Butt, Hans-Jurgen; Michael Kappl; Karlheinz Graff (2006). Physics and Chemistry of Interfaces. Wiley-VCH. ISBN 978-3-527-40629-6
- 14. IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "detergent". doi:10.1351/goldbook.D01643