VOLUME 15 ISSUE 4
WHAT’S HAPPENING AT MISSOURI S&T (FORMERLY UMR):
SHORT COURSE DATES
We will be offering "Introduction to Paint Formulation" May 20-24 (Spring 2019). This course is intended to give the person a fundamental knowledge of how to approach a starting formulation and troubleshoot it. This course involves both lecture and laboratory work.
For more information see our web site at http://coatings.mst.edu and to register contact us at mstformulation@mst.edu or call 573-341-4419. **These courses are held on the Rolla Campus**
ONLINE SHORT COURSE
We are offering "Introduction to the Coating Systems" online short course. This course is targeted for automotive and aviation type OEM companies. This self-paced seminar will cover the painting system from the composition of paints to the evaluation of the dry film. The pigments, resin, solvents and additives will be discussed including their influence on the coatings performance. Color measurement, surface profile, and other evaluation criteria will be related to composition. The importance of surface preparation and other manufacturing criteria will show the system complexity and each step's importance.
We are offering "Surface Defects: Elimination from Human and Process Contaminants" online short course. This course addresses many of the issues in prevention and minimization of defects. The course covers the defects caused by the coatings process, as well as human issues, including personal care product causes. Several of the surface defects are discussed – from basic principles and real world automotive and aircraft examples. The highly practical approach of this course will greatly aid the personnel involved in the painting operation to reduce and systematically approach issues.
EMPLOYMENT TAB
Anyone wanting to have job opening listed, please contact us at (573) 341-4419 or e-mail: mstformulation@mst.edu . You can also write to us at Missouri S&T Coatings Institute, BOM #2, 651 W. 13th St., Rolla, MO 65409-1020. Our web site is http://coatings.mst.edu
TECHNICAL INSIGHTS ON COATINGS SCIENCE
Silver Nanoparticles as Biocides in Coatings
Abbie Braden, Graduate Research Assistant

Image from Polymer Paint Colour Journal5
Coatings that have antimicrobial properties have been used for decades, though with the recent increased interest in nanoparticle technology, there have been developments made in these additives used to fend off bacteria. These coatings are found in hospitals, kitchens, bathrooms, or anywhere that bacteria are abundant and human contact is likely. Not only do these coatings have health benefits, but they are typically more durable, which decreases the frequency of repainting. They also hold aesthetic appeal longer by blocking any sort of staining due to mold.
Antibacterial additives are typically referred to as biocides and are used not only to protect the finished dry film, but also to prevent bacteria growth and fouling of the paint while it is being stored in the can. Biocides can be organic or inorganic materials, usually depending on the amount of UV radiation the paint will be exposed to, the type of bacteria/contamination likely to occur, or the compatibility with other raw materials. Common biocides are comprised of different variants of isothiazolones or zinc oxides, though these are known to be skin irritants and may cause other heath risks. Recently, metal nanoparticles, particularly silver, have been under study as their use as biocides. These nanoparticles have a high surface area and are simple to make through an environmentally and cost friendly process using vegetable oils.3
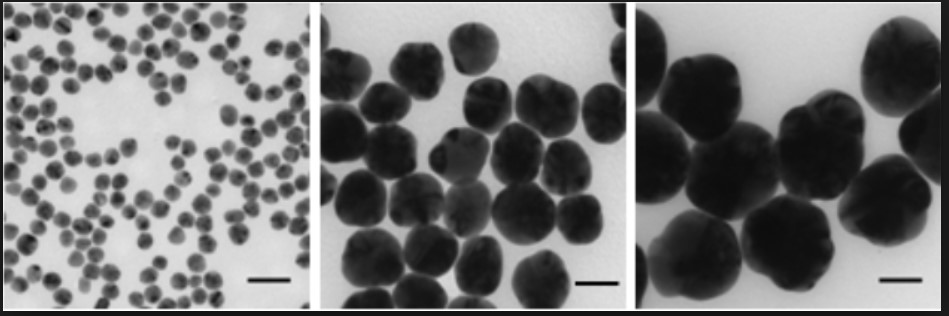
TEM Image of silver nanoparticles from Sigma Aldrich. Scale bar 50nm.6
It is well known that silver ions are formed when silver metal is oxidized, and these ions are very effective antimicrobial materials1. Within the last decade, silver has been applied to every day products such as clothing and toothbrushes to create an extra layer of protection against bacteria1. Silver antibacterial additives are now being included in some paints. Scientists have studied the genes of certain bacteria exposed to silver and determined that the silver ions affect many different biological pathways within the species, hindering reproduction and growth. Uncharged silver nanoparticles have also been seen to cause structural damages to the outer membrane of bacteria and cause degradation1, though the mechanism through which this occurs is still under study. Work done by Morones suggests the size of the nanoparticle must be within 1-10nm for antibacterial properties to be effective. Studies have proven that silver works on both gram negative as well as gram positive bacteria. In addition to the advantage of a higher surface area, these uncharged nanoparticles are also less likely to leach out of the paint system, and therefore do not kill organisms that are not in direct contact with the coating. Silver ions and other organic biocides tend to migrate out of the coating and can have negative effects on the surrounding microorganisms. However, silver nanoparticle effectiveness against fungi is not as good as other organic biocides.4
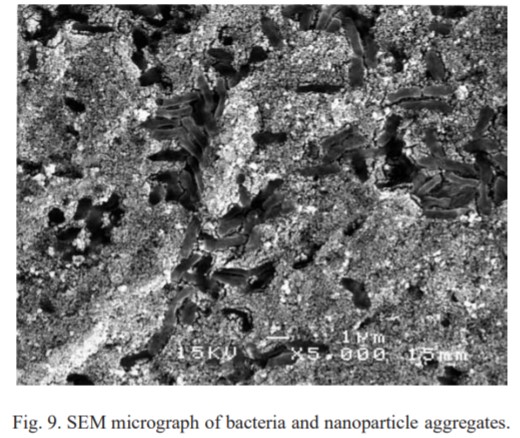
SEM Image of silver nanoparticle aggregations and dead bacteria cells1.
While the long term environmental study of nanoparticles is still under much study and debate, they do indeed have many short term advantages due to their stability inside the film, as well as decreasing the frequency of recoating.
References:
- Sondi, I. & Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 275, 177–182 (2004).
Morones, J. R. et al. The bactericidal effect of silver nanoparticles. Nanotechnology 16, 2346–2353 (2005).- Ashavani Kumar, Praveen Kumar Vemula, Pulickel M. Ajayan & George John. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nature Materials volume7, 236–241 (2008)
- Künniger, T. (2014). Release and environmental impact of silver nanoparticles and conventional organic biocides from coated wooden façades. Environmental Pollution, 184, 464–471
- Roberts, Sally. Biocides Transition in the EU. Polymers Paint Colour Journal. (2018)
- Oldenburg, Steven J. nanoComposix, Inc. Silver Nanoparticles: Properties and Applications. Millipore Sigma. (2019). Retrieved from https://www.sigmaaldrich.com/technical-documents/articles/materials-science/nanomaterials/silver-nanoparticles.html
