VOLUME 15 ISSUE 2
WHAT’S HAPPENING AT MISSOURI S&T (FORMERLY UMR):
SHORT COURSE DATES
We will be offering "Introduction to Paint Formulation" May 20-24 (Spring 2019). This course is intended to give the person a fundamental knowledge of how to approach a starting formulation and troubleshoot it. This course involves both lecture and laboratory work.
We are also offering a new course, "Coatings Composition and Properties for Sales and Marketing Personnel" March 25-27 (Spring 2019). This course is designed for those in the industry who buy and/or sell raw materials into the coatings market, as well as those who buy or sell coatings or simply use coatings. The course is intended to help the newer person in the field gain a better understanding of the science behind paint. For more information, including course times and fees, click the course name above.
For more information see our web site at http://coatings.mst.edu and to register contact us at mstformulation@mst.edu or call 573-341-4419. **These courses are held on the Rolla Campus**
ONLINE SHORT COURSE
We are offering "Introduction to the Coating Systems" online short course. This course is targeted for automotive and aviation type OEM companies. This self-paced seminar will cover the painting system from the composition of paints to the evaluation of the dry film. The pigments, resin, solvents and additives will be discussed including their influence on the coatings performance. Color measurement, surface profile, and other evaluation criteria will be related to composition. The importance of surface preparation and other manufacturing criteria will show the system complexity and each step's importance.
We are offering "Surface Defects: Elimination from Human and Process Contaminants" online short course. This course addresses many of the issues in prevention and minimization of defects. The course covers the defects caused by the coatings process, as well as human issues, including personal care product causes. Several of the surface defects are discussed – from basic principles and real world automotive and aircraft examples. The highly practical approach of this course will greatly aid the personnel involved in the painting operation to reduce and systematically approach issues.
EMPLOYMENT TAB
Anyone wanting to have job opening listed, please contact us at (573) 341-4419 or e-mail: mstformulation@mst.edu . You can also write to us at Missouri S&T Coatings Institute, BOM #2, 651 W. 13th St., Rolla, MO 65409-1020. Our web site is http://coatings.mst.edu
TECHNICAL INSIGHTS ON COATINGS SCIENCE
Determination of the UV damage to paint
Peng Geng and Abbie Braden, Graduate Research Assistants, Missouri S&T Coating Institute
There are many challenges that affect paint durability, sun light is one of them that often cannot be avoided. Fig 1. Shows the electromagnetic spectrum covering electromagnetic waves from 1 x 10-14 to 1 x 104 meters wavelength. Ultraviolet (UV) is an electromagnetic radiation that has a wavelength from 200nm to 400nm, (under 200nm is considered vacuum UV, which is not part of the solar spectra at sea level). This only represents a small portion of the spectrum, however, it constitutes about 10% of the total sea level solar output1.
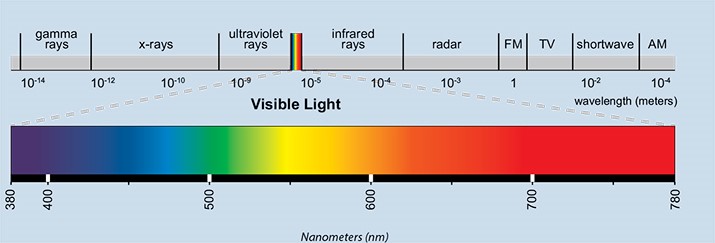
Fig 1. Electromagnetic Spectrum2
UV damage is the most prevalent effect the sun has on coatings. While coatings are constantly being designed to prevent this damage, not all pigments, resins and additives are resistant to UV. In addition to the coating itself, the substrate can also be degraded. For example, wood contains lignin which will be damaged by UV exposure, so the substrate needs a layer of protection from the UV which is the coating. UV can cause electronic excitation, which in turn can cause bonds to break. If a bond breaks heterolytically, it will produce two radicals as depicted below. These radicals can react with oxygen to produce peroxides and hydroperoxides. They can also disproportionate to yield an alkene and an alkane. Other reactions can include cross-linking, hydrogen abstraction and other radical and ionic reactions. Thus, the polymer or molecule can be reduced in molecular weight and /or oxidized to produce color. The cleavage of the polymer chain will generally result in lower tensile strength and result in cracking, increased wear, coating erosion and color change. The oxidative degradation can cause photo-bleaching of pigments and yellowing or color change in the resin or pigment. Occasionally, additives such as a biocide or other highly conjugated molecule becomes colored when photo-oxidized.
R-R -----à R. + R.
R. + O2 ------à R—O---O. ------à R—O—O---R or R—O—O—H
2 R CH2CH2. ------à RCH=CH2 + RCH2—CH3
Over a long period of time, UV exposure will cause paint to crack, fade, age, degrade or lose adhesion. It is important to make sure the pigments, resins and additives are resistant to UV. One example is acrylic resins, they absorb light at about 217nm, so the UV from sun light won’t do damage to it. Aromatics like polystyrene which contain a benzene ring or other polymers that contain an aromatic group will absorb UV light and go to excited state. Upon extended exposure to UV the polystyrene based resin coating will yellow3. Thus, it is critical to know how to determine the damage caused by UV light in daily weathering. Some of the analytical methods are discussed below.
Flexibility
The paint must have proper flexibility and adhesion to prevent cracking when exposed to stress. There are methods used for determining the flexibility/adhesion of organic paints coated on metallic substrates4. By bending several coated panels (both standard and weathered) over a series of different sized conical shaped bars until cracking occurs in the paint film and comparing before and after UV exposed coatings, we can tell if UV damaged the coatings. Impact can also detect damage. If an epoxy primer is top coated with a pigmented acrylic, any UV going through the top coat can damage the epoxy since it is aromatic and highly susceptible to UV damage. This type of damage can occur in automotive coatings and especially when a UV aged coating gets impacted by gravel which results in a chip. Testing by exposure in Arizona is used to evaluate this type of primer surface failure. A micro indenter can also be used to evaluate the modulus of the coating before and after exposure. The Fisher micro indenter can measure the hardness, modulus and the modulus as a function of depth into the coating. Thus if damage is only on the surface the underlying modulus should be the same as the un-exposed panel.
Gloss
Gloss describes the ability of a surface to reflect light in a specular direction. The magnitude of the gloss is angle dependent. Fig 2. Shows the BYK-Gardner Glossmeter. It is calibrated by a black glass standard with a defined refractive index, and measures the specular gloss of substrates at 20°, 60° and 85°geometry6. If the coatings were damaged under UV, the surface may be oxidized or break, which change the surface of the coatings. The comparison of before and after UV exposed samples can show the gloss rating changes.

Fig 2. BYK- Gardner SC-4510 Gloss Meter
Color
Fig 3. is a BYK colorimeter, it expresses color as the L*a*b values, L* presents the lightness, a* presents the green-red color ratio and b* for the blue-yellow ratio7. The changes in these three values from the non-exposed to the UV exposed sample corresponds to the reading ΔL, Δa and Δb from the instrument. By gathering the numerical values of L*a*b, non-exposed coating compared to the coating that has been exposed to UV, any change in the values represents damage caused by the UV exposure. It should be remembered that this is based on reflected light and the observed color is based upon the subtraction of light intensity at different wavelengths. The color shift can give clues as to what has changed in the coating.

Fig 3. BYK-Gardner Color Guide
IR
Infrared radiation (IR) is the electromagnetic radiation with a longer wavelength compared with visible light. Chemical bonds in coatings vibrate when excited by IR at a specific frequency, or wavelength. By running a diffuse or attenuated total reflectance IR spectra on both the unexposed and UV aged coating surface any changes in functional groups in the coating can be observed. It may then be possible to determine any functional groups that have changed.
Inhibitors
UV inhibitors are often used to reduce damage by UV. However, the inhibitors work far better when they are placed in a coating layer above the layer they are to protect. For example, if an inhibitor is placed in the acrylic coating over an epoxy primer the primer will be protected. However, if the inhibitor is placed in the epoxy layer the epoxy will still fail at the acrylic/epoxy interface since it has no inhibitor at the interface. Think of it like a quarterback picking up the ball without a center in front of him. He will be tackled immediately every time. If the center hikes the ball back to the quarterback the center then becomes a defender of the quarterback protecting him. Getting the protection out in front is the key to protection.
It should also be noted that both TiO2and ZnO are both photo oxidation catalysts. If TiO2 is not coated or if the coating gets damaged during dispersion it can result in resin degradation when exposed to UV. ZnO in the presence of moisture and UV generates hydrogen peroxide which can degrade pigments and resins. Both of these pigments should be understood since these issues can come up on occasion.
Remember both coating and substrates can undergo photodegradation. Part of the coatings job is to protect substrates from UV damage. The UV absorption ability of TiO2 is one of the major reasons we paint many substrates white.
References
1. "Reference Solar Spectral Irradiance: Air Mass 1.5". Archived from the original on 27 January 2011. Retrieved 12 November 2009.
2. Eye lighting international.
3. Masayoshi Nakahara: “The Science of Color”, Baifukan (2002), p. 108.
4. ASTM D4145-10.
5. ASTM D523-14.
6. ASTM E1347-06.
7. Lynch, David K.; Livingston, William Charles (2001). Color and Light in Nature (2nd ed.). Cambridge, UK: Cambridge University Press. p. 231. ISBN 978-0-521-77504-5. Retrieved 12 October 2013. Limits of the eye's overall range of sensitivity extends from about 310 to 1050 nanometers.
