VOLUME 14 ISSUE 5
WHAT’S HAPPENING AT MISSOURI S&T (FORMERLY UMR):
SHORT COURSE DATES
We will be offering "Introduction to Paint Formulation" May 20-24 (Spring 2019). This course is intended to give the person a fundamental knowledge of how to approach a starting formulation and troubleshoot it. This course involves both lecture and laboratory work.
We are also offering a new course, "Coatings Composition and Properties for Sales and Marketing Personnel" March 25-27 (Spring 2019). This course is designed for those in the industry who buy and/or sell raw materials into the coatings market, as well as those who buy or sell coatings or simply use coatings. The course is intended to help the newer person in the field gain a better understanding of the science behind paint. For more information, including course times and fees, click the course name above.
For more information see our web site at http://coatings.mst.edu and to register contact us at mstformulation@mst.edu or call 573-341-4419. **These courses are held on the Rolla Campus**
ONLINE SHORT COURSE
We are offering "Introduction to the Coating Systems" online short course. This course is targeted for automotive and aviation type OEM companies. This self-paced seminar will cover the painting system from the composition of paints to the evaluation of the dry film. The pigments, resin, solvents and additives will be discussed including their influence on the coatings performance. Color measurement, surface profile, and other evaluation criteria will be related to composition. The importance of surface preparation and other manufacturing criteria will show the system complexity and each step's importance.
We are offering "Surface Defects: Elimination from Human and Process Contaminants" online short course. This course addresses many of the issues in prevention and minimization of defects. The course covers the defects caused by the coatings process, as well as human issues, including personal care product causes. Several of the surface defects are discussed – from basic principles and real world automotive and aircraft examples. The highly practical approach of this course will greatly aid the personnel involved in the painting operation to reduce and systematically approach issues.
EMPLOYMENT TAB
There are no current job openings available. Anyone wanting to have job opening listed, please contact us at (573) 341-4419 or e-mail: mstformulation@mst.edu . You can also write to us at Missouri S&T Coatings Institute, BOM #2, 651 W. 13th St., Rolla, MO 65409-1020. Our web site is http://coatings.mst.edu
TECHNICAL INSIGHTS ON COATINGS SCIENCE
Polymeric Dispersants
Ashish Zore, Missouri University of science and Technology
The use of polymeric materials in surface coating formulations has been since prehistoric times. Paleolithic humans made cave paintings using metal oxides as pigment and naturally occurring high molecular weight material such as egg white, animal fats as binders. The polymer here acts as a crude dispersant as well as binder for the pigments and substrate (i.e. cave walls) [3].
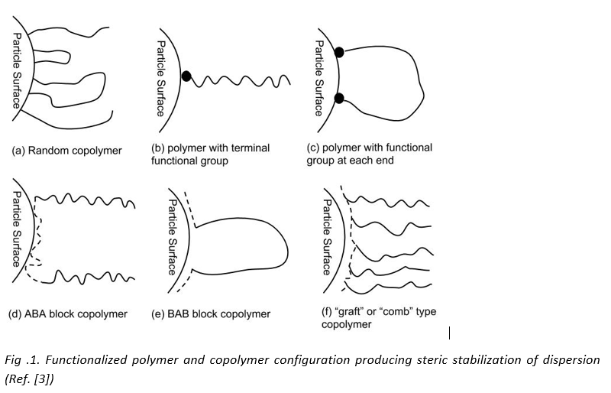
Fundamental design of Polymeric dispersant [3]
The structure of the polymeric dispersant must meet two different requirements: 1) The molecule must contain polymeric chains that give steric stabilization or ionic stabilization in required solvent or resin solution system, and 2) it must be capable strongly adsorbing onto the particle surface. Therefore, copolymers or functionalized polymers can be used as effective polymeric dispersants. They can anchor on to the pigment surface using their functional groups or through association with the polymeric blocks of the copolymer (as shown in the figure below).
Ionic polymeric dispersants are more predominantly used in industry and they provide stability through ionic stabilization i.e. due to repulsion of like charges. The polymers that provides stability through steric stabilization have chain that can either be anchored at one end (as in b, d & f) or at both the ends (as in a, c & e). It has found through studies that chains anchored at one end are most efficient in stabilization [1]. This could be because anchoring at both ends can inhibit the freedom of movement of the chains. Theoretical work [2] and experimental studies have also shown that the molecular weight of the dispersant should not be too high or too low (Ideally 2K to 5K). At low molecular weight the adsorption becomes more reversible.
Advantages of using polymeric dispersants [3]
Polymeric dispersants can reduce pigment-pigment interactions more effectively than conventional dispersants which results in reduced viscosities in pigment containing formulations. Polymeric dispersants are therefore, excellent for reducing the mill base viscosities. The following are the advantages of using polymeric dispersant:
1. Higher Productivity
One can disperse pigments in solution of polymeric dispersant instead of using resin solution. This will allow for much higher concentration of pigment in the millbase at any selected viscosity. This will provide
productivity gains and capital saving through more effective use of dispersing equipment. However, in practice, it is advised to use polymeric dispersant with a dilute resin solution (rather than neat solvent) to prevent shock seeding on letdown. But the productivity and capital gains still apply in most cases. For example, if the pigment concentrate is for use in multiple resin system then using dilute resin solution will not save money since multiple grinds for each resin would be needed.
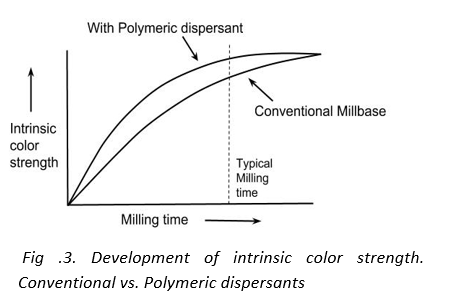
2. Higher color strengths
In pigment based coating systems the intrinsic color strength increases with milling time. This color development is because the aggregate size of the pigment decreases with milling time. However, the color strength tends to reach a plateau when the original primary size of the pigment is reached. The time required to reach the plateau varies from pigment to pigment. For example, phthalocyanine green can develop strength rapidly whereas dioxazine violet develops strength slowly and prolonged milling can give appreciable gain in color strength. It has been observed that polymeric dispersant allow for rapid development of color strength. The milling times required to obtain higher color strength can be considered economically viable.
3. Improved surface coating quality
Polymeric dispersant can increase colloidal stability of the dispersed pigment particles which improves the quality of the final paint. They can Alleviate or eliminate color differences in coating as they offer a way to modify the flocculation/deflocculation balance (Flocculation can shift the color). They ensures the absence of large pigment aggregates in the system thereby improving gloss of the coating. They improve brightness of the coating system by providing better particle size distribution. They improve the flow and reduce the viscosity especially in high pigment concentration systems (high solid paints) due to reduced pigment particle interactions.
The polymeric dispersants should allow the resin system to adhere to the pigment particles because resin is the glue that holds the pigments particles together. If the resin does not adhere to the adsorbed dispersant or to the pigment surface, it can reduce the mechanical properties like the taber abrasion resistance, tensile strength and impact resistance of the paint
References:
- H. L. Jakubauskas, J. Coatings Technol., 58: 71 (1986)
- F. T. Farkas, The industrial paint making process, in Paint and Surface Coating: Theory and Practice (R. Lambourne, Ed.), Ellis Horwood, Chichester, England , p. 309 (1987).
- J. Calbo, Handbook of Coating Additives v.2, Polymeric dispersants, New York, p. 71-103 (1992)