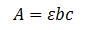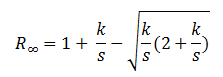Volume 13 issue 6
WHAT’S HAPPENING AT MISSOURI S&T (FORMERLY UMR):
SHORT COURSE DATES
We will be offering "Introduction to Paint Formulation" May 14-18 (Spring 2018). This course is intended to give the person a fundamental knowledge of how to approach a starting formulation and troubleshoot it. This course involves both lecture and laboratory work.
For more information see our web site at http://coatings.mst.edu and to register contact us at mstformulation@mst.edu or call 573-341-4419. **These courses are held on the Rolla Campus**
ONLINE SHORT COURSE
We are offering "Introduction to the Coating Systems" online short course. This course is targeted for automotive and aviation type OEM companies. This self-paced seminar will cover the painting system from the composition of paints to the evaluation of the dry film. The pigments, resin, solvents and additives will be discussed including their influence on the coatings performance. Color measurement, surface profile, and other evaluation criteria will be related to composition. The importance of surface preparation and other manufacturing criteria will show the system complexity and each step's importance.
We are offering "Surface Defects: Elimination from Human and Process Contaminants" online short course. This course addresses many of the issues in prevention and minimization of defects. The course covers the defects caused by the coatings process, as well as human issues, including personal care product causes. Several of the surface defects are discussed – from basic principles and real world automotive and aircraft examples. The highly practical approach of this course will greatly aid the personnel involved in the painting operation to reduce and systematically approach issues.
EMPLOYMENT TAB
We have started an employment section for our students and companies. We have a full time job section, an intern / co-op section and a graduating and alumni students section . Please explore our section on employment on our web site. Anyone wanting to have job opening listed, please contact us at (573) 341-4419 or e-mail: mstformulation@mst.edu . You can also write to us at Missouri S&T Coatings Institute, BOM #2, 651 W. 13th St., Rolla, MO 65409-1020. Our web site is http://coatings.mst.edu
TECHNICAL INSIGHTS ON COATINGS SCIENCE
Hiding
Peng Geng, Missouri S&T Coating Institute
Hide is a very important optical property of paint. It indicates the capacity of paint to hide the surface of an object.
One easy way to measure the hide is to make a drawn down on a black/white card, and then measure the whiteness for the black and white areas of the card. Take the ratio Lb/Lw times 100 is generally termed the hide. The “hide” is film thickness dependent and any comparison must be at a set thickness1.
There are some factors that will affect the hiding, such as the thickness of the paint film as well as the light scattering and light absorption of all the pigments.
Beer-Lambert law is the relationship used for transmittance calculations for transparent samples. It relates the attenuation of light to the material passing through the medium, also, the absorbance of a transparent sample is proportional to the thickness and the concentration. This relationship also holds for reflectance which is what is observed in paint.
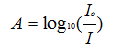
A: absorbance
Io: incident intensity
I: transmitted intensity
C: concentration
B: pathway length
: molar absorptivity
Thickness:
It’s obvious that the thicker paint film, the better hiding ability, since there will be more pigment scattering or absorbing the light.
Light scattering:
Light going through homogeneous material is quite different from going through inhomogeneous medium. Diffuse reflectance was developed to study the common characteristic of internal inhomogeneities materials. The Kubelka-Munk theory describes the propagation of light going through inhomogeneous materials, like paints2.
K: absorption coefficient of the sample
λ: wavelength
s: scattering coefficient
Light scattering is dependent on the particle size and its refractive index difference from the continuous matrix at a specific wavelength.
It is not always true that the larger pigment particle size gives less light scattering. If the pigment particle size is a little smaller than one-half the wavelength of the light, it will give the most light scattering. In that case, pigments that average 200-300 nm in diameter give the highest light scattering, since human eye is most sensitive to yellow-green light, which is around 550 nm3.
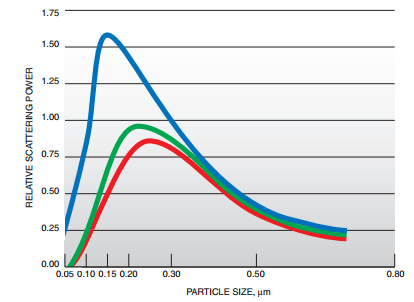
Fig. 1 Comparison of blue, green and red light for particle size vs relative scattering power3
(With permission from Dupont)
Figure 1 shows the particle size vs relative scattering power of blue, green and red light by TiO2 of different particle sizes. It’s obvious that the blue wavelength reaches the maximum relative scattering power when the particle size is around 150 nm, while the green and red are about 200 nm, and the maximum relative scattering power of blue wavelength is much larger than green and red. White pigments must have a consistent size of about 300 nm to minimize the difference in scatter of all wavelengths of light to maintain the highest scattering power. The particle size between 300-400 nm will show the whitest color, and if the particle size is between 100-200 nm, the color will have blue shift.

Fig. 2 Comparison of latex, emulsion and CUP4
Polymer particles suspended in water also exhibit “hide” or opacity. However, this opacity disappears in the paint film due to coalescence of the particles into a film.
Figure 2 is a comparison of Colloidal Unimolecular Polymer (CUP) with larger particles4. The Colloidal Unimolecular Polymer is the polymer formed from a single polymer chain, which was originally dissolved in a good, low boiling solvent, like THF, collapsed into a small sphere when water was added and the solvent, THF, was removed. It’s stabilized by a sufficient number of the ionic or hydrophilic groups on its surface5. Since the CUP particles size ranges from 3-9 nm, they are too small to scatter the light, it is transparent. The particles in an emulsion with 25 nm particles size, produce some light scattering and appears cloudy. Latex particles give a very opaque suspension, because the diameter is typically 100-200 nm. Thus, the water borne suspensions correlate well with Figure 1.
Pigments are defined as organic and inorganic pigments. For scatter, what matters is the difference between reflective index for pigment and reflective index of the medium. Different mediums have different reflective index. Water is 1.33, resins are about 1.5, air is 1.0. Organic pigments have RI all in narrow range around 1.5, which are very similar to resins (RI is 1.4 to 1.5), so almost no scattering occurs. While most inorganic pigments have much higher reflective index, from 1.5 to as high as 5, this is the main reason why we consider most organic pigments as more transparent, but inorganic pigments usually as more opaque6. Table 1 below shows the refractive index of some pigments.
|
Color |
Pigment |
Type |
Refractive Index |
|
Blue |
Lazurite |
Inorganic |
1.50 |
|
|
Indigo |
Organic |
1.49-1.52 |
|
|
Azurite |
Inorganic |
1.73-1.84 |
|
Green |
Chrysocolla |
Inorganic |
1.58-1.60 |
|
|
Volkonskoite |
Inorganic |
2.50 |
|
Yellow |
Gamboge |
Organic |
1.58-1.59 |
|
|
Jarosite |
Inorganic |
1.71-1.82 |
|
|
Goethite |
Inorganic |
2.00-2.40 |
|
|
Yellow iron oxide |
Inorganic |
2.35 |
|
Red |
Realgar |
Inorganic |
2.46-2.61 |
|
|
Red lead |
Inorganic |
2.42 |
|
|
Red iron oxide |
Inorganic |
2.78-3.01 |
|
White |
Chalk |
Inorganic |
1.51-1.65 |
|
|
Titanium Dioxide |
Inorganic |
2.55 anatase 2.73 rutile |
|
|
Zinc Oxide |
Inorganic |
2.00-2.02 |
|
Black |
Carbon Black |
Inorganic |
~1.4-1.57 |
|
|
Black iron oxide |
Inorganic |
2.93 |
Table 1. Refractive index of the pigments7
Absorption:
Human eyes see colors due to the rods and cones of the retina. The eye sees the reflected or scattered light coming from the objects surface including the specular reflectance or gloss. The gloss will contain a percentage of the light coming from the light source. This specular component will have the same color components as the light source. If an object is red, the paint on the surface will absorb all wave length light except red. Black pigment can absorb all wavelengths of visible light, as a result, there is no light reflected or scattered at all, which give 100 % hide. However if the black coating is of high gloss the specular component will still result in the eye observing the light from the source.
All pigments will absorb (K) and scatter (S) light so some extent. This absorption and scatter is different at every wavelength. Thus the scattering and absorption of every pigment at each wavelength of visible light must be known. White pigments like TiO2 have low absorption values but high scattering over the entire visible spectrum and can give high hide of about 98% until the concentration reaches the point where it no longer obeys Beer-Lambert law. A very tiny amount light will pass through to reach the surface, and return through the coating to let people see a shadow of the surface. Adding a trace of carbon black will absorb these photons to make hiding 99.5+%. The addition of the trace of black cannot be seen by the eye, it still looks white. The function of the black pigment is to pick off the few photons who are scattered and spend a long time in the coating, and thus are absorbed. If the photon enters the coating and is scattered out of the paint it will not see the black pigment. This latter path is the dominant one for most photons.
K/S is used by color computer to formulate colored paints. The K/S also defines a pigment contribution to hide at a specific wavelength. K is the absorption of light depending on the colorant and S is the scattering contribution of a dye or pigment 8. The sum of each mixture K/S value equals to the total K/S value.
(K/S)mixture=a(K/S)colorant1 + b(K/S)colorant2 + c(K/S)colorant3 + …. + (K/S)base
K/S is obtained from spectral data, it is calculated for each measured wavelength in the spectrum for a specific pigment blend. This can be applied to use the computer for color matching and color formulating. It can also be used to estimate the expected hide.
Reference:
1. ASTM D4941-06.
2. A, A. Kohanovsky, Journal of Physics D: Applied Physics.
3. DuPont Ti-Pure, titanium dioxide.
4. Minghang Chen, Michael R. Van De Mark, "Rheology studies on colloidal unimolecular polymer (CUP) particles in absence and presence of NaCl," Polymer Preprints (American Chemical Society, Division of Polymer Chemistry), 52(2), 336-337 (2011).
5. Single-Chain Polymer Nanoparticles, V1-05/15/2017, p259-313.
6. K, Schadler, May 2009 issue of The Artist’s Magazine.
7. Henry A. Gardner, George G. Sward, Paint Testing Manual, ASTM International, January 1, 1972, p. 79.
8. Application Note, Vol. 18, No. 7, 2008.