Volume 12 issue 3
What’s Happening at Missouri S&T (formerly UMR):
Short Course Dates
We will be offering "Basic Composition of Coatings" September 28-October 2 (Fall 2015). The Basic Composition course is intended for new personnel in the coatings profession. It targets the components of coatings (resin, pigments, extenders, solvents and additives), testing and specifications, general formulation and manufacturing methods. Basic Composition is primarily a lecture course with several laboratory demonstrations.
We will be offering "Introduction to Paint Formulation" October 26-30 (Fall 2015). This course is intended to give the person a fundamental knowledge of how to approach a starting formulation and troubleshoot it. This course involves both lecture and laboratory work.
For more information see our web site at http://coatings.mst.edu and to register contact us at mstformulation@mst.edu or call 573-341-4419. **These courses are held on the Rolla Campus**
Online Short Course
We are offering "Introduction to the Coating Systems" online short course. This course is targeted for automotive and aviation type OEM companies. This self-paced seminar will cover the painting system from the composition of paints to the evaluation of the dry film. The pigments, resin, solvents and additives will be discussed including their influence on the coatings performance. Color measurement, surface profile, and other evaluation criteria will be related to composition. The importance of surface preparation and other manufacturing criteria will show the system complexity and each step's importance.
We are offering "Surface Defects: Elimination from Human and Process Contaminants" online short course. This course addresses many of the issues in prevention and minimization of defects. The course covers the defects caused by the coatings process, as well as human issues, including personal care product causes. Several of the surface defects are discussed – from basic principles and real world automotive and aircraft examples. The highly practical approach of this course will greatly aid the personnel involved in the painting operation to reduce and systematically approach issues.
Employment Tab
We have started an employment section for our students and companies. We have a full time job section, an intern / co-op section and a graduating and alumni students section . Please explore our section on employment on our web site. Anyone wanting to have job opening listed, please contact us at (573) 341-4419 or e-mail: mstformulation@mst.edu . You can also write to us at Missouri S&T Coatings Institute, BOM #2, 651 W. 13th St., Rolla, MO 65409-1020. Our web site is http://coatings.mst.edu
Technical Insights on coatings Science
The methods for measuring Tg of polymer
Fei Zheng*, Yousef Dawib*, Michael R. Van De Mark
Missouri S&T Coatings Institute
The glass transition temperature (Tg) is an important characteristic parameter of polymers. It is at that temperature that a polymer changes from a glassy state to rubbery state. Measuring Tg is important for polymers because these temperatures usually determine the upper limit for which a plastic can be used and the lower limit temperature of a rubber before it becomes brittle.
At the glass transition temperature, some properties such as specific heat capacity, coefficient of thermal expansion, viscosity, refractive index and elasticity modulus will have an abrupt change, so we could draw a function of those properties versus temperature, then we can see a slope change around the glass transition temperature easily. Accordingly, those methods for measuring polymer physical properties can be employed to determine the Tg of polymers. Let’s take volumetric method, thermo mechanical, differential scanning calorimetry(DSC) and nuclear magnetic resonance(NMR) for example.
Volumetric method
Volumetric method is commonly used to measure Tg. A dilatometer is applied to measure the volume of a polymer sample in its insoluble liquid. The height of liquid level is recorded as the temperature rises. When the turning point appears, at that temperature is the glass transition temperature of that polymer sample. Mercury is often used as the liquid since the polymer is generally insoluble in it.
Thermo mechanical
Thermo mechanical method can measure the stress-strain deformation behavior as a function of temperature. The stress is constant, and stain change increases rapidly at the glass transition temperature. The high temperature provides enough movement to produce free volume for polymer chain segments, which is reflected in the abrupt deformation rate.
DSC
In this instrument, the heat flow into or out of a small amount sample is measured as the sample is subjected to a programmed linear temperature change and heat capacity will be measured. There should be a abrupt slope change at Tg. The method is the most commonly employed to find Tg. An example is shown in figure 1. To increase the sensitivity to Tg modulated DSC can be used which uses a small thermal cycle.
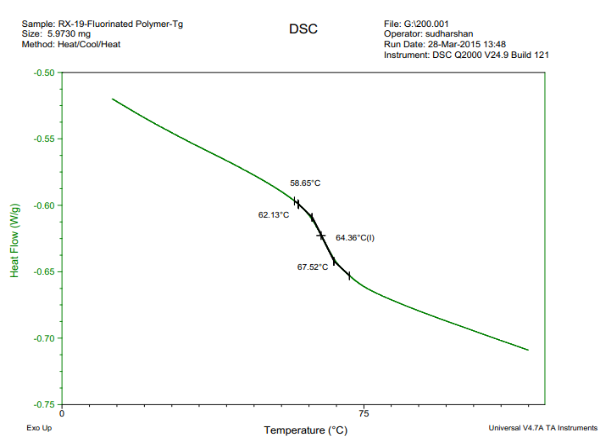
Figure1: Determination of Tg by DSC
NMR
Nuclear magnetic resonance can be also used for measuring Tg. As the temperature increases, the molecular motion increases, as a consequence, random proton orientation distribution increases. At temperature above the glass transition temperature the NMR signal is sharpener than the signal below Tg (1). In addition, NMR technique allows for determination of relaxation time of the protons. In general, the spin-lattice (T1) and spin-spin relaxation time (T2) are measured as a function of temperature. Relaxation rate of protons in a glassy state will be faster than relaxation rate of protons in rubbery state (2).
Solid state NMR has been employed to probe polymer backbone dynamics. Variable temperature 13C Cross polarization (CP) with magic angle spinning (MAS) experiment can be used to assess the motions of the polymer backbone (3),(4). The correlation between 13C NMR spectra linewidth and molecular dynamic has been established. It has been reported that the NMR line width variations arise from molecular motions which vary the lH-13C dipolar interaction (4). McGrath (4) reported 13C NMR spectra of polyisobutylene PIB over the temperature range 140-370 K. The broadening of the quaternary carbon at 38 ppm in the 260-300 K temperature range was attributed to segmental relaxation. See figure 2.
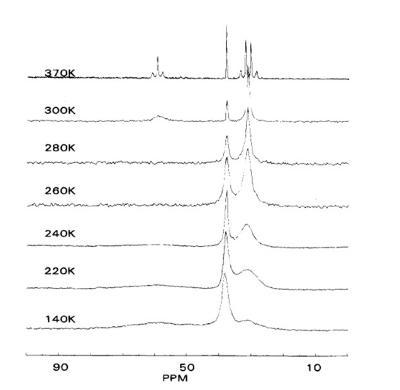
Figure2: 13C NMR spectra of polyisobutylene PIB obtained over the temperature range 140-370 K. The peaks at 6 = 31,38, and 59 ppm correspond to the methyl, quaternary, and methylene carbons, respectively.(4)
From the figure above, you can see that the glass transition temperature falls within a range. The intersection of the slope of two linear lines is usually considered to be the Tg. Different methods may determine different Tgs, however, those results in that range are all acceptable. The rate of temperature change is also important. A lagging effect will occur due to high temperature change rate, which gives you a Tg greater than true value. A study found a linear relationship between Log Tg and the rate of temperature change (5).
For a polymer, its glass transition temperature is not a fixed value. It may change when the measuring method or experimental condition changes. Thus, when you describe the Tg of a polymer, do not forget to indicate your testing method and condition.
References
1- Apperley, David C., Hodgkinson, Paul, Harris, Robin K, Solid-state NMR : Basic Principles & Practice, Momentum Press, LLC, 2012
2- Carmel A. Rubin, John M. Wasylyk, and John G. Baust, J. Agric. Food Chem. 1990, 38, 1824-1827.
3- J. R. Lyeria , C. S. Yannoni, IBM J. Research Development. 1883,Vol. 27 . No. 4.
4- K. J. McGrath, K. L. Ngai, and C. M. Roland, Macromolecules 1996,28, 2825-2830
5- Richeton, J., S. Ahzi, K. S. Vecchio, F. C. Jiang, and R. R. Adharapurapu. "Influence of temperature and strain rate on the mechanical behavior of three amorphous polymers: characterization and modeling of the compressive yield stress." International Journal of solids and structures 43, no. 7 (2006): 2318-2335.
Author information:
Fei Zheng and Yousef Dawib are graduated students in chemistry with the Missouri S&T Coating Institute. Yousef Dawib is completing his Ph.D. This summer and seeking employment.
