Volume 12 issue 2
What’s Happening at Missouri S&T (formerly UMR):
Short Course Dates
We will be offering "Basic Composition of Coatings" March 16-20 (Spring 2015). The Basic Composition course is intended for new personnel in the coatings profession. It targets the components of coatings (resin, pigments, extenders, solvents and additives), testing and specifications, general formulation and manufacturing methods. Basic Composition is primarily a lecture course with several laboratory demonstrations.
We will be offering "Introduction to Paint Formulation" May 18-22 (Spring 2015). This course is intended to give the person a fundamental knowledge of how to approach a starting formulation and troubleshoot it. This course involves both lecture and laboratory work.
For more information see our web site at http://coatings.mst.edu and to register contact us at mstformulation@mst.edu or call 573-341-4419. **These courses are held on the Rolla Campus**
Online Short Course
We are offering "Introduction to the Coating Systems" online short course. This course is targeted for automotive and aviation type OEM companies. This self-paced seminar will cover the painting system from the composition of paints to the evaluation of the dry film. The pigments, resin, solvents and additives will be discussed including their influence on the coatings performance. Color measurement, surface profile, and other evaluation criteria will be related to composition. The importance of surface preparation and other manufacturing criteria will show the system complexity and each step's importance.
We are offering "Surface Defects: Elimination from Human and Process Contaminants" online short course. This course addresses many of the issues in prevention and minimization of defects. The course covers the defects caused by the coatings process, as well as human issues, including personal care product causes. Several of the surface defects are discussed – from basic principles and real world automotive and aircraft examples. The highly practical approach of this course will greatly aid the personnel involved in the painting operation to reduce and systematically approach issues.
Employment Tab
We have started an employment section for our students and companies. We have a full time job section, an intern / co-op section and a graduating and alumni students section . Please explore our section on employment on our web site. Anyone wanting to have job opening listed, please contact us at (573) 341-4419 or e-mail: mstformulation@mst.edu . You can also write to us at Missouri S&T Coatings Institute, BOM #2, 651 W. 13th St., Rolla, MO 65409-1020. Our web site is http://coatings.mst.edu
Technical Insights on coatings Science
Quantum Mechanical Prediction of Corrosion Inhibition Efficiency
Yousef Dawib, Graduate Research Assistant
Missouri University of Science & Technology, Missouri S&T Coatings Institute
Even though inorganic inhibitors are effective compounds in the field of corrosion inhibitors, they have great negative impact on the environment quality and human health. Organic compounds have been used as substitutes for inorganic inhibitors. The effectiveness of organic molecules as corrosion inhibitors is highly dependent on the chemical structure of organic molecules. Experimental investigation of inhibition potential consumes time and money. Computational methods can alternatively provide information about the ability of organic molecule to mitigate the corrosion process. Many studies have correlated quantum chemical parameters to corrosion inhibition efficiencies by the quantitative structure inhibition (activity) relationship (QSAR) approach.
The highest occupied molecular orbital (HOMO), the lowest unoccupied molecular orbital (LUMO), the HOMO–LUMO gap, and the dipole moment (μ) have each been used to characterize inhibitor performance.
The Highest Occupied Molecular Orbital (HOMO):
The HOMO is the highest energy orbital containing electrons. These electrons can be easily donated to metals surface to form coordinating bonds. The organic molecules that have high HOMO energy have a great tendency to donate electrons to unoccupied d orbitals of the metal. The interaction between organic molecule and metal substrate increase the corrosion inhibitive efficiency of organic molecules (1).
Fig.1 shows an example of HOMO electron density distribution of urea and thiourea. The HOMO of Urea has most density located on each pair of nitrogen atoms in amino group whereas the HOMO electron density of thiourea mostly located on sulphur atom in the thiocarbamoyl group. The localization of HOMO electron density on sulphur atom in thiourea increases the HOMO energy level resulting in better inhibition efficiency than urea. This can be explained in terms of the fact that the highest electron density region is generally the preferable site of electrophilic attack. Therefore, a coordinating bond from sulphur atom will be easily formed, rather than nitrogen atoms (1).
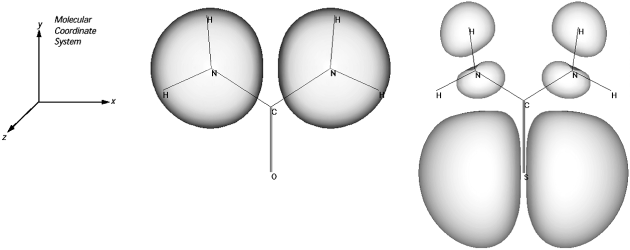
Fig.1. Plot of HOMO electronic density distribution of Urea and Thiourea (1)
The Lowest Unoccupied Molecular Orbital (LUMO):
The LUMO is the lowest energy empty orbital, and is associated with the ability of a molecule to accept electrons. The effective corrosion inhibitor molecule is capable of electrons donating to the unoccupied orbital of the metal along with its ability to accept free electrons from the metal. The probability that the organic molecule would accept electrons from metal would increase if the LUMO energy is low. The inhibition efficiency increases as the ELUMO level decrease (2),(3). It has been reported that effective corrosion inhibitors are usually organic compounds which not only offer electrons to unoccupied orbital of the metal but accept free electrons from the metal (1), (2).
The HOMO–LUMO gap:
The EHOMO–ELUMO energy difference is directly related to the molecule’s stability. A large energy gap results in a high stability during chemical reactions (4). A smaller (EHOMO-ELOMO) gap leads to easier polarization. It has been found that a smaller EHOMO-ELOMO value can enhance corrosion inhibition as a result of strong soft–soft interaction (1), (3).
Dipole moment (µ):
Dipole moment is an indication of an organic molecule’s polarity. Some literature (5) linked the inhibition efficiency to dipole moment by stating that a molecule with low dipole moment has a greater tendency to accumulate on a metal surface, thus has good inhibition performance. However, other studies (2), (6) have found that there was no correlation between the dipole moment and the inhibition efficiency.
References
1- Jian Fang, Jie Li, Journal of Molecular Structure, 593 (2002) 179–185.
2- Guo Gaoa, Chenghao Liang, Electrochimica Acta 52 (2007) 4554–4559.
3- C. Ogretir, B. Mihci, G. Bereket, Journal of Molecular Structure 488 (1999) 223–231.
4- Z. Zhou, R.G. Parr, J. Am. Chem. Soc. 112 (1990) 5720–5724.
5- Gokhan Gece, Corrosion Science 50 (2008) 2981–2992.
6- M. Sahin, G. Gece, F. Karci, S. Bilgic, J Appl Electrochem, 38 (2008), 809–815.
