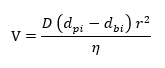VOLUME 17 ISSUE 1
WHAT’S HAPPENING AT MISSOURI S&T (FORMERLY UMR):
ANNOUNCEMENT FOR SECOND SESSION OF SHORT COURSE ON AUGUST 9-13,2021
Due to limited enrollment size and good demand, we have decided to open a SECOND CLASS for Introduction to Formulation Course which will be offered on August 9-13,2021.
The Introduction to Formulation Course offered on August 2-6, 2021 is almost at full capacity and will be conducted as scheduled. We are encouraging all participants to be vaccinated before the course to aid in maintaining a safe course. We are limiting the course to 15 participants due to space constraints. We have moved back into Schrenk Hall and the course lecture and lab will all be in Schrenk Hall. We can still be reached by email at,, formulation@mst.edu, phone 573-341-4882. We wish that everyone stay safe and healthy. Please keep up with changes on our web site. Michael Van De Mark, Director Missouri S&T Coatings Institute. It should be noted that all courses are subject to changes in university restrictions for Covid-19.
ONLINE SHORT COURSE
We are offering "Introduction to the Coating Systems" online short course. This course is targeted for automotive and aviation type OEM companies. This self-paced seminar will cover the painting system from the composition of paints to the evaluation of the dry film. The pigments, resin, solvents and additives will be discussed including their influence on the coatings performance. Color measurement, surface profile, and other evaluation criteria will be related to composition. The importance of surface preparation and other manufacturing criteria will show the system complexity and each step's importance.
We are offering "Surface Defects: Elimination from Human and Process Contaminants" online short course. This course addresses many of the issues in prevention and minimization of defects. The course covers the defects caused by the coatings process, as well as human issues, including personal care product causes. Several of the surface defects are discussed – from basic principles and real world automotive and aircraft examples. The highly practical approach of this course will greatly aid the personnel involved in the painting operation to reduce and systematically approach issues.
EMPLOYMENT TAB
Anyone wanting to have job opening listed, please contact us at (573) 341-4419 or e-mail: mstformulation@mst.edu . You can also write to us at Missouri S&T Coatings Institute, BOM #2, 651 W. 13th St., Rolla, MO 65409-1020. Our web site is http://coatings.mst.edu
TECHNICAL INSIGHTS ON COATINGS SCIENCE
Flooding and Floating Phenomena
Ashish Zore, Research Assistant, Missouri S&T Coating Institute, Missouri University of Science and Technology
Flooding or floating phenomena is defined as the change of color that takes place after application of paint film when the pigment is still wet. It is caused when one or more pigments of the pigment mix gets concentrated on the surface due to migration. Floating appears after application as cells and streaks on the film surface. Flooding is a uniform change of color of the wet film during drying[1].
There are three primary causes of flooding and floating:
- Currents that occur during the drying of the pain
- Variation in the mobility of the different pigments
- Flocculation of one of the pigments in the mix
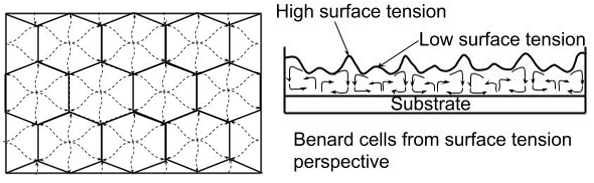
After application of the paint film, the solvent evaporation caused differences in temperature, surface or interfacial tension and density and as a result eddy currents are formed, likely due to osmotic forces. Due to the evaporation of solvents at the film surface, there is an increase in the viscosity and at the same time reduction in surface temperature due to the loss of the heat of vaporization. There is also an increase in the specific gravity and often the surface tension of the materials on the surface. These may act as contributors to the color changes but only in a very minor way. As the solvent evaporates the concentration of chemicals at the surface increases relative to the paint closer to the substrate. The solvent in the lower layers of paint will be forced to migrate toward the surface to dilute the higher concentration. This effect is osmotic flow and will increase in thicker layers of paint, also at higher temperatures and air flow where evaporation is faster. This results in localized eddy currents which form a network of irregular hexagons with touching edges known as Benard cells. The source point is the middle of the cell and the paint sinks at the edges of the cell [2]. This phenomenon occurs in clear matted systems as well as pigmented lacquers and paints. This flow is more pronounced in paints systems of low viscosity and higher film thickness and becomes weaker as viscosity increases and the film thickness decreases. The pigments present in the lacquer get caught up in this turbulent flow and carried along. If the various pigments present have different mobilities then it can lead to separation and therefore to floating or flooding.
The difference in mobility of the pigment can arise due to several factors such as particle size, degree of flocculation, wetting, specific gravity, aspect ratio and electric charge. The main cause behind the difference in mobility of the pigment is the particle size/Aspect ratio and density. The degree of flocculation can be considered as increase of pigment particle diameter and can also change the color. The movement of pigments in the drying film can be related to stokes law for velocity of spherical particle in liquid [3]:
Where V is the Velocity; D, a proportionality constant; dpi, the density of pigment; dbi, the density of binder; r, the radius of the pigment particle; η, the viscosity of the dispersion. It is clear from the above equation pigment diameter is more important than the specific gravity. Some data for relative mobility of the different pigments is mentioned in Table 1 [4]. Due to the difference in the magnitude (as mentioned in Table 1) of mobility of the pigment, the constituent pigments will separate out in the currents that arise during drying.
|
Table 1. Relative mobility of some Pigments |
|||
|
Pigment |
Mobility |
Density g/cc |
Particle size, nm |
|
Titanium dioxide |
2 – 10 |
4.23 |
300-550 |
|
Prussian blue |
500 |
1.83 |
100 |
|
Carbon black |
100000 |
1.1-1.8 |
13-50 |
Floating is caused by one of the pigments in the mix migrating uniformly to the surface. Although, it should be noted that flocculation by itself will often uniformly reduce the color intensity even if no movement up or down occurs. Flocculation in a paint is a not an ideal or good formulation and needs to be addressed to better stabilize the formulation. Flocculation is not flooding but if a pigment flocculates in the can it will reduce its migration aptitude.
The differential mobility of pigments and separation of pigments can cause the color of the dry film to change when the drying process is accelerated using tools like hair dryer, etc. Shading of a coating made in a paint store often use tools like a hair dryer to speed up the drying process of the drawdowns when preparing a shade for a customer. This is done to reduce the wait time for the customers. However, this speed drying also increases the osmotic flow and creates more effect of the differential mobility. The result is different shade than standard. This difference is then corrected by the store to match with the standard and then sold to the requesting customer. Now, when the customer applied the given tinted paint at home it doesn’t match with the standard because it is ambiently dried at home instead of speed drying (as in store). These errors in color matching at store (speed drying) versus the home (ambient drying) is due to differential mobility of pigments. As seen from Table 1, Carbon black pigment has a very high mobility and therefore moves more to the surface than any other pigments. Hence, color shades that are made using black tints can be a nightmare for color matching especially when using speed drying methods.
Low boiling (fast evaporating) solvents like acetone will also speed up the osmotic flow (more color difference) as compared to high boiling (slow evaporating) like EB Solvent (ethylene glycol monobutyl ether). Another factor affecting the mobility is the aspect ratio of the pigments. For e.g., mica has a platy structure and will move faster than if it were a sphere of the same density and weight. The high surface area structure makes the movement faster in the solvent flow just as the sails on the ship makes them move faster by catching more wind. Fumed silica and flattening agents also move faster due to their dendritic structures giving them higher surface area. Hence, a small amount of flattening agent is enough to cause a significant flattening as most of it ends up at the surface where it is needed.
The problem of differential mobility can be solved by using a proper thixotropic agent to adjust viscosity and/or by using a slow evaporating solvent. The latter will also slow the drying of the film.
References:
- Calbo, Handbook of Coating Additives, Adhesion promoters, New York, p. 281-294 (1992)
- Jebsen-Marwedel, Farbe Lack, 314 (1960)
- Kresse, Farbe Lack, 111 (1966)
- H. Bell, J. Oil Color Chem. Assoc., 35: 386 (1952)