VOLUME 16 ISSUE 3
WHAT’S HAPPENING AT MISSOURI S&T (FORMERLY UMR):
SHORT COURSE DATES
We will be offering "Introduction to Paint Formulation" May 18-22 (Spring 2020). This course is intended to give the person a fundamental knowledge of how to approach a starting formulation and troubleshoot it. This course involves both lecture and laboratory work.
For more information see our web site at http://coatings.mst.edu and to register contact us at mstformulation@mst.edu or call 573-341-4419. **These courses are held on the Rolla Campus**
ONLINE SHORT COURSE
We are offering "Introduction to the Coating Systems" online short course. This course is targeted for automotive and aviation type OEM companies. This self-paced seminar will cover the painting system from the composition of paints to the evaluation of the dry film. The pigments, resin, solvents and additives will be discussed including their influence on the coatings performance. Color measurement, surface profile, and other evaluation criteria will be related to composition. The importance of surface preparation and other manufacturing criteria will show the system complexity and each step's importance.
We are offering "Surface Defects: Elimination from Human and Process Contaminants" online short course. This course addresses many of the issues in prevention and minimization of defects. The course covers the defects caused by the coatings process, as well as human issues, including personal care product causes. Several of the surface defects are discussed – from basic principles and real world automotive and aircraft examples. The highly practical approach of this course will greatly aid the personnel involved in the painting operation to reduce and systematically approach issues.
EMPLOYMENT TAB
Anyone wanting to have job opening listed, please contact us at (573) 341-4419 or e-mail: mstformulation@mst.edu . You can also write to us at Missouri S&T Coatings Institute, BOM #2, 651 W. 13th St., Rolla, MO 65409-1020. Our web site is http://coatings.mst.edu
TECHNICAL INSIGHTS ON COATINGS SCIENCE
Adhesion Promoters in Coatings
Ashish Zore, Graduate student, Missouri University of Science and Technology
Adhesion promoters, as the name implies, are additives used to improve the adhesion of polymers to the metal oxide on the metal surface and/or the pigments including extenders. The presence of any polar functional group in a compound can contribute to improved bonding to the mineral surface. However, a successful adhesion promoter must have a balance of characteristic like polymer reactivity, covalent or ionic (in case of amine or carboxyl functional) bonding to inorganic surfaces, hydrolyzability, and other important properties such as surface activity and wetting control [1]. Only a handful of organofunctional silanes meet these requirements.
In 1940, fiberglass reinforced organic resins were used to make glass filled composites materials. These materials had very good strength but their properties deteriorated with time due to loss of bonding between glass and the resin. This bonding problem remained unsolved until mid-1950 when the plastic industry started using a trace amount of organofunctional silanes to improve the adhesive properties of the composite material. The coating industry, in late 1940s, was using organosilicon additives like polydimethylsiloxanes to reduce pigment floating and silking in paints. However, an even greater problem was that of adhesion since coating would peel with prolong exposure of heat, cold, moisture and chemical attack. This problem was solved with the development of organofunctional silanes for use as adhesion promoters in coatings [2].
Adhesion promoter or coupling agents have dual reactivity i.e. it reacts with both polymer and the mineral component forming a durable covalent bond across the interface. These bonds are hydrolysable but can re-form and therefore provide a means of stress relaxation at the organic/inorganic interface which results in improved adhesion and durability [3]. Several coating system like urethane, epoxy, PVC plastisol, acrylic and latex coatings use silane adhesion promoters.
Silane coupling agents are organosilicon monomers that have a general formula: X3Si(CH2)nY, where n=0-3, Y is an organofunctional group selected for reactivity with a given resin, and X is an easily hydrolyzable group, which in many cases is methoxy or acetoxy, that reacts with the surface hydroxyl (OH) groups or water. Some commercial silane (ethoxy/methoxy) coupling agents are listed in Table 1.
Table 1. Representative commercial silane coupling agents used in protective coatings [4]
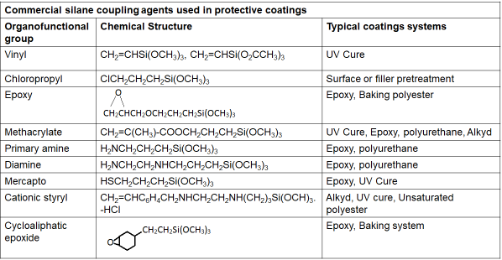
The performance of the coupling agent depends on its interaction with the inorganic (metal oxide) surface and organic polymer (resin). The “receptive” inorganic surfaces which are characterized by the presence of hydroxyl groups (formed due to oxidation) attached to certain elements like Si, Al, Ti and Fe interact strongly with adhesion promoter by forming stable covalent bonds. Adhesion promoters do not interact well with “non-receptive” surfaces like graphite, boron, alkali and alkaline earth oxides as they cannot form stable covalent bonds with silanols. When water is present, the coupling agent will first convert to reactive silanols by hydrolysis. The hydrolysis reaction is as follows:
RSiY3 + H2O à RSi(OH)3 (silanol) + HY (usually an alcohol, Y= OCH3, OC2H5) …….. (I)
The reactive silanols (X = OH) form hydrogen bonds with the hydroxyl group of the substrate and later condenses to form siloxane or O-Si-O bonds [5]. The reaction is as follows:
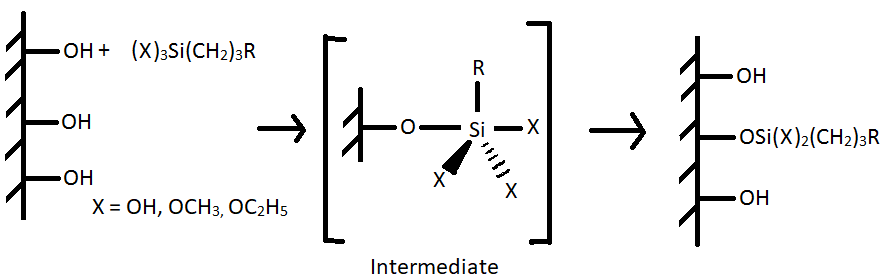 (II)
(II)
In the absence of water, the coupling agent (X = OCH3, OC2H5 directly react as shown in reaction II. The coupling agent can also form polymer (III) which can interact with the metal by adsorption followed by reaction with the hydroxyl group to form siloxane bonds (as in reaction II).
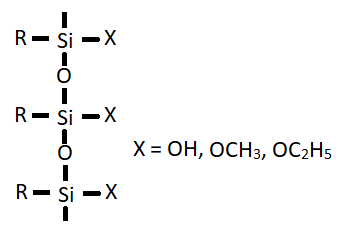 (III)
(III)
For better performance of the silane adhesion promoters, a few precautions are necessary. Silanes are easily hydrolyzed. The paint components should therefore be dry and solvents should be carefully selected [6]. Fillers or pigments can absorb or react with the silane and result in loss of adhesion. This problem can be solved by using excess silane or inert filler like barium sulfate [7]. In case of coating, strong adhesion between resin and pigment/filler particles is very important as it affects properties like tensile strength of the coating. Adding excess silane ensures good adhesion between the resin and pigment/filler particles as well as between the resin and substrate surface. For latex systems, precautions must be taken to ensure that silanes do not migrate into the latex particles. This can be controlled by using silanes that preferentially remain in aqueous phase or by dissolving them in a plasticizer [4].
Reference:
- Silane Coupling Agents, Dow Corning Brochure No. 23-012A-81.
- J. Calbo, Handbook of Coating Additives, Adhesion promoters, New York, p. 281-294 (1992)
- Collins, W., Silanes, Modern Plastics Encyclopedia, 167-168 (1979-1980).
- Plueddemann, E. P., “Silane Adhesion Promoter in Coatings”, Progress in Organic coatings, 11:3 (1983), 297.
- Plueddemann, E. P., Silane Coupling agents. New York: Plenum Press, 1982.
- Walker, P. J., Oil Color Chem. Assoc., 65:11 (1982) 436-443.
- Plueddemann, E. P., “Silane Adhesion Promoter/Polymeric Coatings, in Adhesion Aspect of Polymeric Coating, ed. by K. L. MiHal. New York: Plenum, 1983, p. 363.
