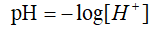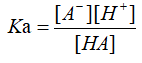VOLUME 16 ISSUE 2
WHAT’S HAPPENING AT MISSOURI S&T (FORMERLY UMR):
SHORT COURSE DATES
We will be offering "Introduction to Paint Formulation" Oct 21-25 (Fall 2019). This course is intended to give the person a fundamental knowledge of how to approach a starting formulation and troubleshoot it. This course involves both lecture and laboratory work.
For more information see our web site at http://coatings.mst.edu and to register contact us at mstformulation@mst.edu or call 573-341-4419. **These courses are held on the Rolla Campus**
ONLINE SHORT COURSE
We are offering "Introduction to the Coating Systems" online short course. This course is targeted for automotive and aviation type OEM companies. This self-paced seminar will cover the painting system from the composition of paints to the evaluation of the dry film. The pigments, resin, solvents and additives will be discussed including their influence on the coatings performance. Color measurement, surface profile, and other evaluation criteria will be related to composition. The importance of surface preparation and other manufacturing criteria will show the system complexity and each step's importance.
We are offering "Surface Defects: Elimination from Human and Process Contaminants" online short course. This course addresses many of the issues in prevention and minimization of defects. The course covers the defects caused by the coatings process, as well as human issues, including personal care product causes. Several of the surface defects are discussed – from basic principles and real world automotive and aircraft examples. The highly practical approach of this course will greatly aid the personnel involved in the painting operation to reduce and systematically approach issues.
EMPLOYMENT TAB
Anyone wanting to have job opening listed, please contact us at (573) 341-4419 or e-mail: mstformulation@mst.edu . You can also write to us at Missouri S&T Coatings Institute, BOM #2, 651 W. 13th St., Rolla, MO 65409-1020. Our web site is http://coatings.mst.edu
TECHNICAL INSIGHTS ON COATINGS SCIENCE
pH in coatings
Peng Geng, Graduate Research Assistant, Missouri S&T Coating Institute
pH is known as the hydrogen ion concentration index. It is a scale of hydrogen ion activity in solution, which is the measure of the degree of acidity and alkalinity of the solution in the usual sense1. There is not a certain explanation for the letter p and H, however, most people believe that pH stands for potential of hydrogen since this was measured using potential differences, and it represents the negative of the base 10 logarithm of the hydrogen ion concentration2.
At 25°C, when the pH is <7, the solution is acidic; when the pH is >7, the solution is alkaline; and when the pH is 7, the solution is neutral3. The acidity and alkalinity of the aqueous solution can also be measured by pOH, which is the negative logarithm of the hydroxide ion concentration. Due to the self-ionization equilibrium in the water, pH + pOH =14 at 25°C. A pH of less than 7 indicates that the concentration of H+ is greater than the concentration of OH-, so the solution is acidic, while a pH of higher than 7 is the opposite. Therefore, the smaller the pH, the stronger the acidity of the solution; the higher the pH, the stronger the alkalinity of the solution. In addition, for some very strong acids, the pH can be less than 0 and for very strong base, the pH can be greater than 14. Under non-aqueous or non-standard temperature and pressure conditions, pH=7 may not mean that the solution is neutral, which requires determining the pH to neutral by calculating the ionization constant of the solvent under these conditions4.
Water is a very weak electrolyte that can undergo weak ionization. The equation is: 2H2O?H3O++OH-, abbreviated as H2O?H++OH-, which is an endothermic process5. It was found that only 1×10-7 mol of water molecules were ionized in 1L of pure water at 25°C. The number of H+ and OH- ionized by water molecules is always equal in all cases. At 25°C, [H+]=[OH-]=1×10-7 mol/L. The dissociation constant is described as [H+]*[OH-]=Kw, it only changes by temperature. Ka is acid dissociation constant, it is the equilibrium constant for an acid-base reaction6. The general equation can be written as:
Where HA is an acid that dissociates into the conjugate base A-, and a hydrogen ion H+. In general, the logarithmic constant pKa is used instead of Ka for practical purpose.
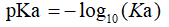
Table 1. pKa of some compouds as example
(Advanced organic chemistry, Jerry March, Second Ed, 1977, McGraw-Hill, Ch8, p227)
|
Acid strength |
Acid |
Base |
Approximate pKa (relative to water) |
|
Strong |
FSO3H |
FSO3- |
Too strong to measure |
|
Strong |
H2SO4 |
HSO4- |
-9 |
|
Strong |
HCl |
Cl- |
-7 |
|
Strong |
RCH2OH2+ |
RCH2OH |
-2 |
|
Strong |
H3O+ |
H2O |
-1.74 |
|
Strong |
HNO3 |
NO3- |
-1.4 |
|
Strong |
HSO4- |
SO4- |
1.99 |
|
Weak |
RCOOH |
RCOO- |
4-5 |
|
Weak |
NH4+ |
NH3 |
9.24 |
|
Weak |
ArOH |
ArO- |
8-11 |
|
Weak |
H2O |
OH- |
15.74 |
|
Weak |
RCONH2 |
RCONH- |
17 |
|
Weak |
RCH2OH |
RCH2O- |
18 |
|
Very poor |
ArNH2 |
ArNH- |
25 |
|
Very poor |
ArCH3 |
ArCH2- |
35 |
|
Very poor |
CH4 |
CH3- |
40 |
In general, a larger Ka value (or smaller pKa value) represents a stronger acid because of the greater ability to dissociate at the same concentration. The acid concentration, conjugate base, proton and hydroxide ions can be easily calculated using the dissociation constant. Usually, acids with a pKa value of less than -2 can be considered as strong acids. The pKa of some compounds are shown in Table 1.
Buffer solution refers to a mixed solution composed of a weak acid and its conjugate base, or a weak base and its conjugate acid, which can reduce the influence of added acid or strong base on pH of the solution, which keeps the pH value relatively stable7. Several of these buffers are used as calibration standards, since their pH are more constant. For a buffer solution system, pKa can be expressed as:
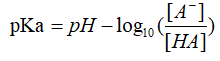
A pH indicator is commonly used to visually determine the acidity or basicity of a solution, and is used in finding the pH titration endpoint. The indicator is an organic weak acid or weak base, which can partially ionize into ions and hydrogen ions, their moelcules and ions will show different colors in solutions when protonated or deprotonated8. Some common pH indicators are phenolphthalein, methyl orange, and 4-nitrophenol. Phenolphthalein is colorless in acidic and neutral solution, but shows pink color in base. Methyl orange is red when pH is less than 3.1, orange between 3.1 and 4.4, and when the pH is higher than 4.4, it will turn yellow. 4-nitrophenol is colorless below pH 5.4 and yellow above pH 7.5.
pH paper is a low cost and relatively accurate way to determine the pH value. pH paper is made by soaking paper in a pH indicator solution and then dried. There are variety of pH papers available, they differ by ranges and sensitivity. For general uses, pH paper has a range of 1 to 12. For waterborne paints, the pH paper with a range of 8 to 9.5 is often used.
A pH meter is a scientific instrument measuring the hydrogen ion activity in solution, determining its acidity or alkalinity expressed as pH. The main measuring components of a pH meter are a measuring electrode and the reference electrode9. The measuring electrode is sensitive to pH, and the potential of the reference electrode is stable. When two electrodes are in the same solution, the only thing that matters is potential difference between the measuring and reference electrodes. By measuring the change of potential of measuring electrode, the pH of the solution can be obtained. In order to get precise measurement, pH meter should be calibrated before each measurement. Calibration needs to be performed with at least two standard buffer solutions that have different pH values bracketing the expected value10.
For coatings, pH is a very important factor that has several major effects on coatings. First, any change of the pH, may result in the change of the viscosity. One example is a cellulose thickener, increasing the pH of the formulation will create ionic groups on the polymer chain, which will increase the “a” value in Mark Houwink equation, due to ionic repulsion along the polymer chain. Second, pH can vary the pigment dispersion stability. Pigments, at the isoelectric point, there is no charges. The net charge on the pigment surface are affected by pH and become more positive or negative due to the gain or loss of protons. Al high pH the negatively charged pigments repel each other, giving a better dispersion and increasing the viscosity. If the pH is lowered both negative and positively charged pigments may coexist and will attract each other causing flocculation. Third, pH can also accelerate the rate of hydrolysis, lower the molecular weight and thus the tensile strength and hardness11. When adding base to an acrylic latex or resin, the ester which is not part of the polymer backbone can be hydrolyzed. The hydrolysis produces ionic groups along the chain and increasing the ionic repulsion increasing the viscosity. However, if the esters are in the backbone of a polymer, such as in a polyester, the hydrolysis would cleave the polymer reducing the molecular weight. This bond cleavage between the carbonyl and oxygen, will decrease the molecular weight and lose hardness as well as potentially reduce viscosity. The final major issue is the issue of the corrosive effect of pH at very low and high values. When an aqueous solution has a pH of less than 2, or more than 12.5 it will be considered corrosive12. The pH of coatings is normally around 7-8.5, however, if the pH is higher than 9.5, it should be labeled as corrosive to skin.
Reference:
- Bates, Roger G. Determination of pH: theory and practice. Wiley, 1973.
- Lim, Kieran F. (2006). "Negative pH Does Exist". Journal of Chemical Education. 83 (10): 1465. Bibcode:2006JChEd..83.1465L. doi:10.1021/ed083p1465.
- Francl, Michelle (August 2010). "Urban legends of chemistry". Nature Chemistry. 2 (8): 600–601. doi:10.1038/nchem.750. ISSN 1755-4330.
- Trummal, Aleksander; Lipping, Lauri; Kaljurand, Ivari; Koppel, Ilmar A.; Leito, Ivo (2016). "Acidity of Strong Acids in Water and Dimethyl Sulfoxide". The Journal of Physical Chemistry A. 120(20): 3663–3669. doi:10.1021/acs.jpca.6b02253. PMID 27115918.
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "autoprotolysis constant". doi:10.1351/goldbook.A00532
- Bisswanger, Hans (2008). Enzyme Kinetics: Principles and Methods (PDF). Weinheim: Wiley-VCH. p. 302. ISBN 978-3-527-31957-2.
- Petrucci, Ralph H.; Harwood, William S.; Herring, F. Geoffrey (2002). General Chemistry (8th ed.). Prentice Hall. pp. 667–8. ISBN 0-13-014329-4.
- Schwarzenbach, Gerold (1957). Complexometric Titrations. Translated by Irving, Harry (1st English ed.). London: Methuen & Co. pp. 29–46.
- "pH meter". Encyclopædia Britannica Online. 2016. Retrieved 10 March 2016.
- Galster, Helmuth (1991). pH Measurement: Fundamentals, Methods, Applications, Instrumentation. Weinheim: VCH Publishers, Inc. ISBN 978-3-527-28237-1.
- Dr. Van De Mark. The important of pH in waterbornes. Coatings r&d notebook, Sep 25, 2003.
- California hazardous waste classification.