VOLUME 15 ISSUE 5
WHAT’S HAPPENING AT MISSOURI S&T (FORMERLY UMR):
SHORT COURSE DATES
We will be offering "Introduction to Paint Formulation" Oct 21-25 (Fall 2019). This course is intended to give the person a fundamental knowledge of how to approach a starting formulation and troubleshoot it. This course involves both lecture and laboratory work.
For more information see our web site at http://coatings.mst.edu and to register contact us at mstformulation@mst.edu or call 573-341-4419. **These courses are held on the Rolla Campus**
ONLINE SHORT COURSE
We are offering "Introduction to the Coating Systems" online short course. This course is targeted for automotive and aviation type OEM companies. This self-paced seminar will cover the painting system from the composition of paints to the evaluation of the dry film. The pigments, resin, solvents and additives will be discussed including their influence on the coatings performance. Color measurement, surface profile, and other evaluation criteria will be related to composition. The importance of surface preparation and other manufacturing criteria will show the system complexity and each step's importance.
We are offering "Surface Defects: Elimination from Human and Process Contaminants" online short course. This course addresses many of the issues in prevention and minimization of defects. The course covers the defects caused by the coatings process, as well as human issues, including personal care product causes. Several of the surface defects are discussed – from basic principles and real world automotive and aircraft examples. The highly practical approach of this course will greatly aid the personnel involved in the painting operation to reduce and systematically approach issues.
EMPLOYMENT TAB
Anyone wanting to have job opening listed, please contact us at (573) 341-4419 or e-mail: mstformulation@mst.edu . You can also write to us at Missouri S&T Coatings Institute, BOM #2, 651 W. 13th St., Rolla, MO 65409-1020. Our web site is http://coatings.mst.edu
TECHNICAL INSIGHTS ON COATINGS SCIENCE
Absolute Molecular Weight in GPC
Ashish Zore, Graduate Research Assistant, Missouri S&T Coating Institute
Molecular weight of the polymer determines its mechanical properties. Higher molecular weight polymer have strong durable mechanical properties but at the same time have high viscosity and poor processability. The control of molecular weight and molecular weight distribution is necessary to improve the desired physical properties of the polymer. Of all the techniques [1] used for measuring the molecular weight, the most commonly used method is Gel permeation chromatography (GPC) or Size exclusion chromatography (SEC). SEC separates polymer chains of different size using a SEC column packed with porous material. Molecules of smaller size are trapped in the pores more often than molecules of larger size. Smaller molecules therefore, spend longer time in the column and elute out later as compared to larger molecules. The elution time of the molecule corresponds to its size [2].
Deriving molecular weight
The accuracy of the molecular weight in a GPC instrument depends on the type of detectors present in the system. The refractometer is used to determine the concentration of the solution as well as the refractive index increment (dn/dc – change in refractive index with change in concentration). The viscometer is used for determination of size (Rg – Radius of gyration) and structure (from the Mark-Houwink exponent ‘α’ as discussed under Universal calibration section) and the Light scattering detector can give the molecular weight and the size (Rg – Radius of gyration). GPC instruments with all three detectors give absolute molecular weight but are also the most expensive. There are three different ways to measure molecular weight in GPC – Conventional calibration, Universal calibration and Triple detector [3].
1. Conventional Calibration
Conventional calibration require only an RI detector (refractometer). The column is calibrated with a set of polymer standards which are usually polystyrene. The molecular weights obtained here are relative to the calibrant used. If a polyethylene sample is run in a column calibrated with polystyrene standard, the molecular weight obtained would be incorrect for polyethylene. This is because the column separates on the basis of molecular size (i.e. the Rg – Radius of gyration) and not molecular weight. Two different polymers of identical molecular weight can have different size in the same solvent because they interact differently with the solvent. Even though the molecular weights are relative, this is acceptable for many people who simple interested in in comparing the molecular weights of an unknown against a set of acceptable values. The instrument configuration required for this method is also low cost.
2. Universal Calibration
Universal Calibration was introduced by Benoit, et. al. where instead of plotting the log molecular weight of a series of narrow standards vs. retention (as in conventional calibration), the log of the product of the intrinsic viscosity [η] and molecular weight M is plotted vs. retention. Universal calibration require a viscometer and an RI detector (refractometer). The product of intrinsic viscosity [η] and molecular weight M is related to hydrodynamic volume and it was found that the values of hydrodynamic volume for a variety of narrow standards resulted in a singular (hence the term “Universal”) calibration curve. We can also plot the log of the intrinsic viscosity vs. the log of the molecular weight which is called as the Mark-Houwink plot and get the get the values of α and K of the Mark-Houwink equation, [η] =KMα. The values of α and K are for different polymer/solvent combinations are available in Polymer Handbook. One can obtain the absolute or accurate molecular weight using these empirical constants in many of the commercial GPC software packages available today, provided the values are correct. The universal calibration is widely used to obtain accurate molecular weights of random coil type polymer which represent majority of the polymers analyzed today. This method is also able to determine the extent of branching relative to the linear standard. The viscometer is also useful for determining the size of the molecule (as the radius of gyration, Rg) in the solution. The intrinsic viscosity and the molecular weight can be used to calculate the Rg using the Ptitsyn-Eizner modification of the Flory-Fox equation [3].
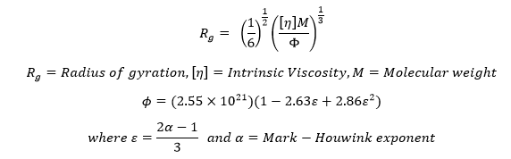
One of the limitations of this method is that there should be no interaction between the eluent or column packing material. Also, Rod, sphere or globular shaped (such as protein) polymer configurations do not behave well with the Universal method.
3. Triple detector
The triple detector has all three detectors – Refractometer, Viscometer and Light Scattering. Light scattering technique is used to determine the molecular weights of the polymer solution without the use of any calibration standards. In fact, the molecular weight can be obtained from the online light scattering instrument connected in series before the refractometer and viscometer. The interaction of light with the polymer molecule induces a temporary dipole moment which oscillates in phase with the incident beam. The molecule re-radiates the light in all directions due to Rayleigh scattering. The excess light scattering intensity compared to the solvent background caused by the presence of polymer molecules is directly proportional to the molecular weight and sample concentration [4,5]. Light scattering is measure at different angles to get better accuracy for different polymer configurations.
Malvern sells SEC instruments with triple detector array (TDA) unit which have the three detectors- Refractometer, Viscometer and Light scattering. It also provides an option for UV detector for measuring the concentration. The Light scattering detector used in the TDA measures scattering at two angles – RALLS (Right angle laser light scattering) and LALLS (low angle laser light scattering). Right angle scattering provides optimum signal to noise ratio performance as it is least affected by problems of stray light, cell window reflections, air bubbles and particle contamination [6]. Low angle scattering is used for accurate determination of molecular weights of odd shaped species (like rod). The presence of both the light scattering detectors allows accurate determination of molecular weights for a wide range of polymer conformations. Wyatt sells SEC instruments with multiple detectors but with an option of having separate modules for each of the detectors. Their light scattering module has Multi-Angle static Light Scattering (MALS) detector for absolute characterization of the molar mass and size of macromolecules and nanoparticles in solution. It measures static light scattering at 18 different angles. An important point to remember when selecting an instrument is the distance between the detectors. This distance can induce broadening of peaks and create errors. The less dead volume in the lines, lower is the broadening. All instruments must compensate for the dead volume.


Fig. 1. Malvern’s Viscotek system with Triple detectors (Top left), Wyatt’s MALS (Multi angle light scattering) module DAWN (Top right)*, Wyatt’s refractometer module Optilab (bottom left)* and Wyatt’s viscometer module ViscoStar* (*With the permission from Wyatt Technology).
References:
- F.W. Billmeyer, Jr., Textbook of Polymer Science, 3rd edn. (Wiley, New York, 1984)
- W.-F. Su, Principles of Polymer Design and Synthesis, 2013, XIII, 306 p, 480.
- Chi-San Wu, Handbook Of Size Exclusion Chromatography And Related Techniques, V 91, 2004.
- P. Kratochvil, Classical light scattering from polymer solutions, Elsevier, Amsterdam, 1987.
- P. Kratochvil, Light scattering of polymer solutions, M.B. Huglin (Ed.), Academic press New York, 1967, Ch. 7.
- Data presented in “Model 305 TDA detector Instrument Manual”, Man0420-1.0, Rev 1.35, April 2009, Malvern company.