Volume 4 Issue 2
What’s Happening at Missouri S&T:
Summer 2007 Short Course - "Introduction to Coatings Composition and Specifications"
** Register Today - Some Spaces Still Available!!!**
This summer we will be offering "Introduction to Coatings Composition and Specifications" July 16-18, 2007 , in St. Louis Missouri, a course designed for the new coatings person in areas such as sales, marketing or production . The course was initiated by a number of raw material companies and distributors requesting a course with this format. This course is not as heavily technical as is our “Basic Composition of Coatings¿? and “Introduction to Paint Formulation¿? courses. The ?Introduction to Coatings Composition and Specifications?? course is a two and a half day course which will discuss the types of coatings, the basic composition of coatings and the tests and specifications used by the industry. This course will allow the participant to gain the fundamentals needed to work in this industry and to communicate more clearly. More information can be found at the above links, on our website at http://coatings.mst.edu , or by emailing coatings@mst.edu or calling 573-341-4419.
Fall Short Course Dates
This fall we will be offering “Basic Composition of Coatings¿? September 10-14, 2007 and. The Basic Composition course is intended for new personnel in the coatings profession. It targets the components of coatings (resin, pigments, extenders, solvents and additives), testing and specifications, general formulation and manufacturing methods. Basic Composition is primarily a lecture course with several laboratory demonstrations. For more information see our web site at http://coatings.mst.edu and to register contact Michael Van De Mark at coatings@mst.edu or call 573-341-4419. **This course is held on the Rolla campus**
This fall we will be offering “Introduction to Paint Formulation¿? October 22-26, 2007 . This course is intended to give the person a fundamental knowledge of how to approach a starting formulation and troubleshoot it. This course involves both lecture and laboratory work. For more information see our web site at http://coatings.mst.edu and to register contact Michael Van De Mark at coatings@mst.edu or call 573-341-4419. **This course is held on the Rolla campus**
Kia Alavi receives Missouri S&T Chemistry Department's Outstanding Alumnae Award
 |
Kiarash Alavi Shooshtari (Kia Alavi) Received the Outstanding Alumnae Award from the Chemistry Department. The award was presented by Dr. Van De Mark, Director of the Missouri S&T Coatings Institute and Dr. Shooshtari’s Ph.D. advisor.
Kia Alavi was born in Tehran, Iran. In 1990, he joined Missouri S&T as an undergraduate. He finished his BSc on December of 1994. He obtained his PhD in chemistry under supervision of Dr. Michael Van De Mark. He graduated May 2000 and worked at Missouri S&T as a Senior Research Specialist. He Joined TMT-Pathway in Salem, Oregon as a Senior Research Chemist on May 2001 and became Technical Director by August 2001. He joined Johns Manville in Denver, Colorado as a Senior Research Chemist in June of 2004 and became Research Associate/Sectional Manager in 2007. He currently has 21 patent applications with Johns Manville from which five have been published. His areas of expertise are organic, polymer synthesis and coatings science.
Technical Insights on Coatings Science
Atom Transfer Radical Polymerization - Introduction and Application in Coatings for Synthesis of Block Polymers
By Vishal Patil, Missouri S&T Graduate Student
Abstract:
In recent times there has been a significant rise in the use of high solids, low VOC coatings. Typical chain polymerization systems, both radical and ionic, proceed with accompanying chain breaking reactions because of the type of propagating centers/ or the reagents present. The bimolecular termination and chain transfer in these systems limit the lifetime of propagating radicals. So chain polymerization with control i.e. without chain-breaking reactions would be highly desirable, since they would allow the synthesis of block copolymers by the sequential addition of different monomers. This kind of polymerization, known as controlled living radical polymerization is very useful in the synthesis of resin systems, since the desired copolymers can be effectively made with a better control on molecular weight and hence on viscosity of the resin.
Introduction:
Free radical polymerization is widely used in industry because of the availability of a wide range of radically polymerizable monomers, mild reaction conditions and tolerance to impurities such as moisture. There are several methods by which controlled radical polymerization are carried out. Atom transfer radical polymerization (ATRP) is one of the popular methods. Radical generation in ATRP involves an organic halide undergoing a reversible redox process catalyzed by a transition metal compound such as a cuprous halide. ATRP proceeds as described in equations 1-1 through 1-3.
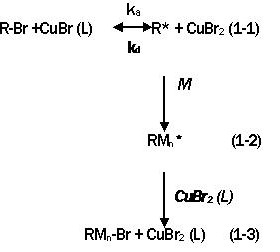 |
Where L is a ligand that complexes with the cuprous salt and helps to solubilize it in the organic reaction system. ka and kd are rate constants for activation and deactivation of the halide initiator, with k=ka/kd.
Activation of the initiator involves CuBr metal center undergoing an electron transfer with simultaneous halogen atom abstraction and expansion of its co-ordinate sphere. R* is the reactive radical that initiates polymerization. CuBr2 (L) is the persistent (metallic) radical that reduces the steady state concentration of propagating radicals and minimize normal termination of living polymer. The initiator and persistent (metallo) radical are also called activator and deactivator, respectively.
The studies of Matyjaszewski et al., 2001, established the free radical nature of ATRP. ATRP is similar to conventional radical polymerization as far as the effects of inhibitors and retarders, solvents and chain transfer agents are concerned. The regioselectivity, streoselectivity and copolymerization behavior are also analogous.
Kinetics of ATRP:
The concentration of radical [R*] in ATRP can be obtained as
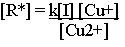 |
from the equilibrium expression for eq 1-1.
The rate of propagation and therefore the rate of polymerization is the sum of many individual propagation steps. Since the rate constants for all propagation steps are the same, one can express the polymerization rate by,
Rp = kp[M*][M]
where, [M] is the monomer concentration and [M*] is the total concentration of chain radicals, that is, all radicals of size Mi and large.
Combining Eq (1-4) with the expression for propagation (Eq- 1-5) we have the polymerization rate as
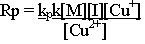 |
Where [I] is the initiator, RBr in this case. The polymerization rate is expressed as the change in monomer concentration with time.
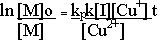 |
An efficient ATRP requires fast and quantitative initiation (activation of RBr) so that all propagating species begin growth at the same time which results in a narrow molecular weight distribution. Rapid reversible deactivation of propagating radicals is needed to maintain low radical concentration and minimize normal termination of living polymers. This further ensures narrow molecular weight distribution because all propagating chains grow at the same rate and for the same length of time. The number average degree of polymerization for a living polymerization under these conditions is given by
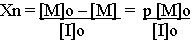 |
Where [M]o and [I]o are initial concentrations of monomer and initiator, respectively. [M] and p are the monomer concentrations and fractional conversion of monomer at any time in the reaction.
Effects of Components on ATRP:
A typical ATRP involves various components such as the monomer, an initiator with a (pseudo) halogen, and a catalyst (composed of a transition metal species with any suitable ligand). Sometimes an additive is used. For a good ATRP, other factors such as solvent and temperature, must also be considered.
Typical monomers include styrene, (meth)acrylates, (meth)acrylamides, and acrylonitrile, which contain substituents that can stabilize the propagating radicals. The activator catalyst is a transition metal in its lower oxidation state. The deactivator is a transition state metal in its higher oxidation state, either formed spontaneously or deliberately added. Successful ATRP of a specific monomer requires matching the various components so that the dormant species concentration exceeds the propagating radical concentration by a factor of ~ 106.
A host of organic halides are used as initiators, some of the common being alkyl halides like chloroform and carbon tetrachloride, allyl and benzyl halides, a- haloesters, a -haloketones, a -halonitriles and sulfonyl halides. Fast and quantitative initiation is needed to achieve narrow molecular weight distributions, but an initiator that decomposes too rapidly results in increased normal bimolecular termination of propagating radical. Bromides are more reactive than chlorides, iodides are highly reactive but undergo light indices side reactions and fluorides are unreactive. The initiator reactivity should be matched to the monomer reactivity. This is achieved by using a halide with an organic group similar in structure to the propagating radical, for example, benzyl halide, a -haloester and a -halonitrile for styrene, acrylate and acrylonitrile respectively.
The metal catalyst used must have two oxidation states easily accessed by a one electron transfer, an affinity for halogen, an expandable co-ordination sphere to accommodate the halogen, and a strong complexation with the ligand. Catalysts based on copper are the most used because they are useful irrespective of the monomer. A metal catalyst functions in presence of appropriate ligands. Ligands solubilize the transition metal salt in organic media and adjust the redox potential of the metal center for appropriate activity. For copper catalysts, bidentate and multidentate, but not monodentate nitrogen ligands work best. Bridged and cyclic ligands as well as branched aliphatic polyamines yield more active catalysts than do simple linear ligands. 4,4-Dialkyl-2,2’-bipyridine and tris-(2-dimethylaminoethyl)amine are examples of active ligands.
ATRP has been carried out in bulk, solution, suspension and aqueous emulsion. Solvents used for solution polymerization include toluene, ethyl acetate, alcohols, water, ethylene carbonate, DMF and supercritical CO2. Temperature for ATRP are generally in the range 60-120°C, with some polymerizations proceeding at lower temperatures. The polymerization rate increases with increased temperature because both kp and k(=ka/kd) increase with increasing temperature.
Depending on the monomer, the other components of the system, as well as the reaction conditions need to be adjusted, so that radical concentrations are sufficiently low to effectively suppress normal termination. The less reactive monomers such as ethylene, vinyl chloride, and vinyl acetate, have not been polymerized by ATRP. Acidic monomers such as acrylic acid are not polymerized because they interfere with the initiator by protonation of the lignands. The carboxylate salts of acidic monomers are polymerized without difficulty.
Reverse ATRP:
ATRP process discussed up to now is normal ATRP which used RX and a transition metal in its lower oxidation state to establish the equilibrium between dormant and propagating species. Reverse ATRP involves generating the same ATRP system by using a combination of a conventional radical initiator such as AIBN and a transition metal in its higher oxidation state (for example Cu2+[Wang and Matyjaszewski, 1995 b]). The initiator generates radicals, which react with Cu2+ to establish the equilibrium with RX and Cu+.
ATRP allows the synthesis of well defined polymers with molecular weights up to ~ 150,000-200,000. At higher molecular weights, normal bimolecular termination becomes significant especially at very high conversion and results in a loss of control. There also appears to be slow termination reactions of Cu2+ with both the propagating radicals and polymeric halide.
Block Polymers by ATRP:
There are two methods by which block copolymers are synthesized via ATRP; the one pot sequential and isolated macro-initiator method. In one pot sequential method an AB diblock copolymer is produced by polymerizing monomer A. Monomer B is then added when most of A has reacted. In the isolated macro-initiator method, the halogen terminated poly A (RAnX) is isolated and then used as an initiator (the macro initiator) together with CuX to polymerize monomer B. RAnX is usually isolated by precipitation with a non solvent or some other technique. The halogen terminated macro initiator is typically quite stable and can be stored for long periods prior to use in forming the second block. Usually the reaction is carried out to 90% conversion because the normal bimolecular termination steps in as the monomer concentration falls. This is true for both methods.
The one pot sequential method has the disadvantage that the propagation of the second monomer involves a mixture of the second monomer plus unreacted first monomer. The second block is actually a random copolymer. The isolated macro initiator method works best, since it avoids the ‘contamination’ of the second block.
Examples:
US patent 6762263 [Callais, Peter A, et al] discusses a method to form block polymers for use in high solids coatings.
7.34g of MONAMS (monoalkoxyamine-5) and 20g of ethyl benzene are charged to a 100 ml jacketed stirred glass reactor equipped with thermocouple, reflux condenser and nitrogen. A mixture of monomer is prepared separately consisting of 52g butyl acrylate, 20 g 2-hydroxyethyl acrylate, 8g styrene. Once the reactor and contents reach 120°C, monomer mix is pumped continuously into the reactor at a constant rate of 0.22g/min; such that the reactor feed time is 5 hours. After monomer addition is complete, the polymerization reaction is allowed to continue for an additional 8 hours at the same temperature. The progress of the reaction is monitored by sampling at regular intervals. At the end of the reaction time the reactor is cooled to ambient temperature, the resin is collected by standard techniques and submitted for residual monomer analysis by gas chromatography and molecular weight analysis. After one hour post feed, total residual monomer was 9.9% by weight, Mn was 3500 and molecular weight distribution was 1.2. At the end of the 8 hour period, total residual monomers were 0.9% by weight, Mn was 3900 and molecular weight distribution was 1.4.
Conclusion:
ATRP offers good possibilities to control chain topology. The catalytic system is crucial in degree of control. Halide exchange offers degree of freedom in rate/ polydispersity. Ligands of catalyst influence course of reaction significantly. ATRP is a useful method in synthesis of well defined block polymers for use in high solids, low VOC coatings.
References:
1. Matyjaszewski, K, Chemical reviews 2001,101,2921-2990.
2. Hong, S.C. Macromolecules 2002, 35, 7592-7605.
3. Principles of Polymerization, 4th edition, by George Odian.
4. Polymer Chemistry, 3rd edition, by Malcolm Stevens.
Is there a topic you would like discussed? Contact us by e-mail at coatings@mst.edu.
| September 10-14, 2007 Basic Composition of Coatings This course provides an overview of the components of paint and how they work. Participants are also introduced to methods for testing and manufacture of paint. |
| October 22-26, 2007 Introduction to Paint Formulation This course provides techniques used in formulating paint from raw materials. It involves formulating and making paint in the laboratory, "Hands on!" July 16-18, 2007 Introduction to Coatings Composition and Specifications This two and a half day course is designed for the new coatings person is fields such as sales, marketing or production. |
To subscribe/unsubscribe to this newsletter, click here. Feel free to forward this to your colleagues. |
