Missouri S&T Coatings Institute Newsletter Volume 5 Issue 3
What’s Happening at Missouri S&T:
(formerly UMR)
On January 1, 2008 the University of Missouri-Rolla (UMR) , changed its name to Missouri University of Science and Technology (Missouri S&T) . The new name more accurately describes the university’s true focus. Missouri S&T was founded in 1870 as the first technological university west of the Mississippi River. The original name was Missouri School of Mines and Metallurgy. In 1964 the university was re-named the University of Missouri-Rolla in an effort by the state of Missouri to consolidate the names of the four campuses which form the University of Missouri system. The name change does not change the excellent educational programs at Missouri S&T but helps students identify the university as a technological and science based campus. Missouri S&T has been increasing its enrollment over the past 5 years from less than 5,000 to over 6,000 students and a has planned an increase to over 7,000. The UMR Coatings Institute is now the Missouri S&T Coatings Institute.
**All our web sites and e-mail addresses have also changed. In the past they were @UMR.EDU but now they are @MST.EDU. Our UMR domain and e-mail addresses have now been retired so please update your records accordingly with our new e-mail (coatings@mst.edu) and web address, http://coatings.mst.edu/. Our university's new spam filter is also fairly aggressive, so if you e-mail us and do not get a response within a few days, please e-mail again or call us.
Fall Short Course Dates
This fall we will be offering ?Basic Composition of Coatings" September 15-19, 2008. The Basic Composition course is intended for new personnel in the coatings profession. It targets the components of coatings (resin, pigments, extenders, solvents and additives), testing and specifications, general formulation and manufacturing methods. Basic Composition is primarily a lecture course with several laboratory demonstrations. For more information see our web site at http://coatings.mst.edu/index.html and to register contact Michael Van De Mark at coatings@mst.edu or call 573-341-4419. **This course is held on the Rolla campus**
This fall we will be offering ?Introduction to Paint Formulation" November 3-7, 2008. This course is intended to give the person a fundamental knowledge of how to approach a starting formulation and troubleshoot it. This course involves both lecture and laboratory work. For more information see our web site at http://coatings.mst.edu/ and to register contact Michael Van De Mark at coatings@mst.edu or call 573-341-4419. **This course is held on the Rolla campus**
Technical Insights on Coatings Science
Sources and Benefits of Thixotropy in Paints
By Joseph Kellogg, Undergraduate Chemistry Major, Missouri S&T Coatings Institute
Thixotropy is one type of non-Newtonian fluid behavior, similar to, and often confused with shear thinning. A thixotropic fluid will experience a decrease in viscosity not only as a function of shear stress, but also as a function of time. When the shear is removed, the viscosity increases, also as a function of time, until it returns to its resting viscosity. It is when this time-dependent component becomes insignificant that the fluid approaches true pseudoplasticity.[1]
Figure I. Time Dependence of Viscosity of Thixotropic and Shear Thinning Fluids
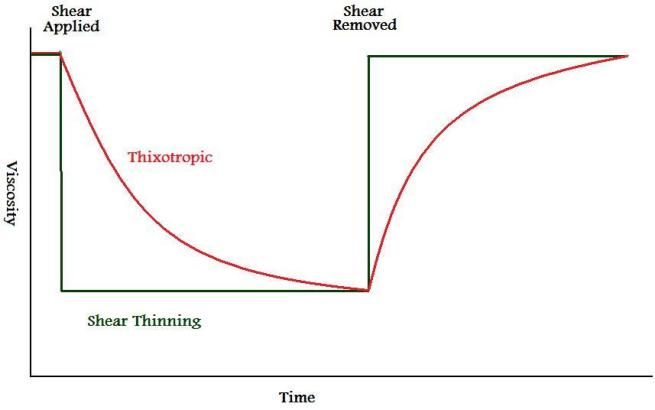 |
Thixotropy is important for coatings because the viscosity will increase after the shear from application ceases to prevent sag, while staying low long enough to allow good flow and leveling. It also helps to prevent settling of pigments.[2]
The basic explanation for the presence of thixotropy is the formation of weakly bonded particle networks. When shear force is applied, this network is gradually disrupted, decreasing the viscosity. While all thixotropic fluids contain networks, the type of network can vary. In paints that contain clay, the plate-like particles bear charges in such a way that the edges of one plate are attracted to the face of another. This results in a network reminiscent of a house of cards, trapping the liquid inside the structure.[1]
Some finely divided particles, such as silicas and some pigments, can have solvent adsorb onto their surfaces, effectively increasing the particle size. These larger particles can then interact with each other and form a “pearl-chain” structure. Other types of networks can be formed with cellulosic thickeners used to stabilize pigments, or from the resin itself, such as in polyamide modified alkyds, which structure themselves via hydrogen bonding between the amide groups.[1]
One widely used additive for imparting thixotropy is silica. For this purpose, fumed silica is formed by burning silicon tetrachloride in the presence of hydrogen and oxygen, which yields a very high purity, amorphous silica. Because the thixotropic properties of particles like silica arise from the adsorption of solvent molecules, a higher surface area tends to lead to higher shear resistance, and faster recovery times upon the removal of the shear force. By heating a silica sol in acetone above the critical point before degassing, a very high surface area is obtained on the silica. This colloidal silica has been used with a number of different solvents and resins.[1]
Different surface treatments can result in either hydrophobic or hydrophilic silica particles. For resins such as unsaturated polyesters, either can be used. However, for hydrogen bonding resins such as vinyl esters, the hydrophilic silica particles are stabilized by the resin, and cannot produce a network.[3]
When clays are used, they are usually in the form of their sodium or quaternary ammonium salts. These ammonium salts can be substituted with organic groups, making the clays more compatible with organic solvents.[1] A study of laponite clay by Martin et al. showed that the clay particles were organized into micro-domains, which then joined to form macroscopic super-aggregates, which in turn would interact with each other to form the network. However, the recovery time for these mixtures were less than 1 second without the addition of a peptizer to reduce the electrostatic attraction between particles.[4]
Some organic metal complexes are also used to impart thixotropy to paints, especially aluminum and titanium chelate complexes. The di- and triethanolamine complexes of titanium are water soluble, and thus are very effective for water-based latex emulsions. However, the emulsion must be stabilized by a colloid such as a cellulose ether to form the hydrogen bonds needed for the thixotropic structure. However this colloid and the chelate can be used in smaller amounts with resins that contain small amounts of acetlyacetate groups. Also, these titanium chelates tend to improve color and water resistance to the final paint.[5]
Hydrogenated castor oil can also be used, although there are a number of issues regarding its use. To produce the necessary structure, it must be heat activated, which can lead to problems if done improperly. When mixed with a solvent, it tends to separate out into two phases upon storage. If a binder polymer is added to make it more stable, it loses its versatility as an additive. Also, these systems tend to display a dependence on rheological history, and can require several weeks of rest to reset. Some systems have been developed that combine hydrogenated castor oil with amide waxes or sugar-aldehyde derivatives. The derivatives of sugar alcohols and other hydrocarbons can also be used, often incorporated into the resin.[5]
Some isocyanates, urethanes, and ureas are also known to provide thixotropic structure. Many of these thixogens are used for coatings that must be cured, either chemically or thermally. Of the modifications to the resin itself, the most common is probably the incorporation of a polyamide into an alkyd resin. These tend to provide good flow and leveling for high-gloss applications. However, care must be taken when using organic pigments, as their interactions with the resin may interfere with the hydrogen bonding and creation of the thixotropic structure.[5]
References
[1] Walton, A. J. Techniques for Producing Thixotropic Paints etc.: Part 1. Pigm. Resin Technol. 1982, 11, 4-9.
[2] Wicks, Z. W.; Jones, F. N.; Pappas, S. P.; Wicks, D. A. Organic Coatings: Science and Technology, 3rd ed.; Wiley: Hoboken, NJ, 2007.
[3] Barthel, H.; Dreyer, M.; Gottschalk-Gaudig, T.; Litvinov, V.; Nikitina, E. Fumed Silica – Rheological Additive for Adhesives, Resins, and Paints. Macromol. Symp. 2002, 187, 573-84.
[4] Martin, C.; Pignon, F.; Piau, J. M.; Magnin, A.; Lindner, P.; Cabane, B. Dissociation of Thixotropic Clay Gels. Phys. Rev. E: Stat. Phys, Plasmas, Fluids. 2002, 66.
[5] Walton, A. J. Techniques for Producing Thixotropic Paints etc.: Part 2. Pigm. Resin Technol. 1982, 12, 4-10.
