Volume 14 Issue 1
WHAT’S HAPPENING AT MISSOURI S&T (FORMERLY UMR):
SHORT COURSE DATES
We will be offering "Introduction to Paint Formulation" May 14-18 (Spring 2018). This course is intended to give the person a fundamental knowledge of how to approach a starting formulation and troubleshoot it. This course involves both lecture and laboratory work.
For more information see our web site at http://coatings.mst.edu and to register contact us at mstformulation@mst.edu or call 573-341-4419. **These courses are held on the Rolla Campus**
ONLINE SHORT COURSE
We are offering "Introduction to the Coating Systems" online short course. This course is targeted for automotive and aviation type OEM companies. This self-paced seminar will cover the painting system from the composition of paints to the evaluation of the dry film. The pigments, resin, solvents and additives will be discussed including their influence on the coatings performance. Color measurement, surface profile, and other evaluation criteria will be related to composition. The importance of surface preparation and other manufacturing criteria will show the system complexity and each step's importance.
We are offering "Surface Defects: Elimination from Human and Process Contaminants" online short course. This course addresses many of the issues in prevention and minimization of defects. The course covers the defects caused by the coatings process, as well as human issues, including personal care product causes. Several of the surface defects are discussed – from basic principles and real world automotive and aircraft examples. The highly practical approach of this course will greatly aid the personnel involved in the painting operation to reduce and systematically approach issues.
EMPLOYMENT TAB
We have started an employment section for our students and companies. We have a full time job section, an intern / co-op section and a graduating and alumni students section . Please explore our section on employment on our web site. Anyone wanting to have job opening listed, please contact us at (573) 341-4419 or e-mail: mstformulation@mst.edu . You can also write to us at Missouri S&T Coatings Institute, BOM #2, 651 W. 13th St., Rolla, MO 65409-1020. Our web site is http://coatings.mst.edu
TECHNICAL INSIGHTS ON COATINGS SCIENCE
Rheology of Coatings Part I
Abbie Braden, Graduate Research Assistant, Missouri S&T Coating Institute
Rheology
One of the most important properties to consider within the paint and coatings realm is rheology. Rheology is the study of how a material flows and reacts to forces exerted by surroundings such as pipes, or mixer blades. Understanding this behaviour of a coating can indicate the best methods for application, storage (1), and tinting, among other things.
Viscosity
Perhaps the most well-known rheological property is viscosity, or the resistance a material has to flow. This property is determined by a variety of things in coatings, such as molecular weight, concentration of solvents, temperature, shear, and even time. If the conditions are on one end of the spectrum (high molecular weight, low solvent concentration...), the material will tend to flow slowly and therefore have a higher viscosity versus a material on the opposite side that is more willing to flow easily. In coatings, viscosity has a major effect on the method of application. When the coating is intended to be applied using a sprayer, a lower viscosity is needed in order for the material to be atomized in a way that will produce an even coat with the desired thickness. Usually, a higher viscosity coating will produce a thicker film, although it is more likely to exhibit an “orange peel” defect. Viscosity can also affect the production of the coating. Higher viscosity paints are more prone to generating heat when undergoing mixing, and must be more closely monitored than thinner paints that can dissipate heat at a faster rate.
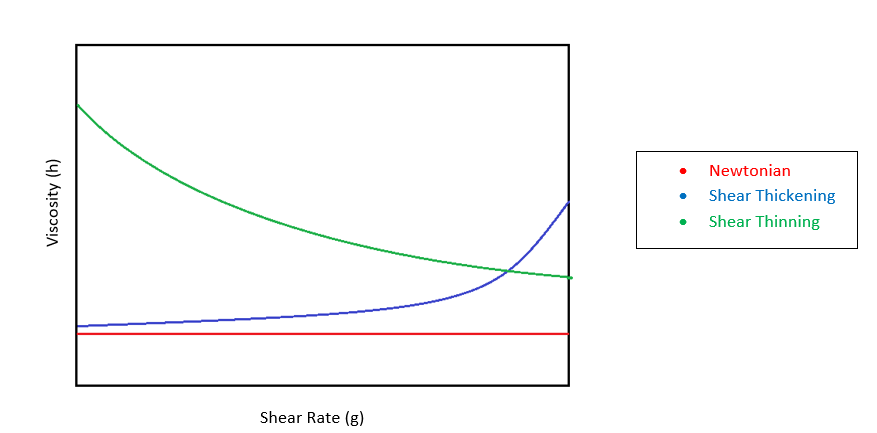
There are two different main types of fluid materials that are considered in the coatings world; Newtonian, and Non-Newtonian. For Newtonian fluids, viscosity is represented as a liner relationship between shear stress and shear rate (2) and can be defined using the equation τ=ηγ where τ represents shear stress, η is viscosity, and γ is shear rate. Viscosity of Newtonian fluids is dependent only on temperature. Some examples of Newtonian fluids include water, most solvents, and some polymer suspensions.
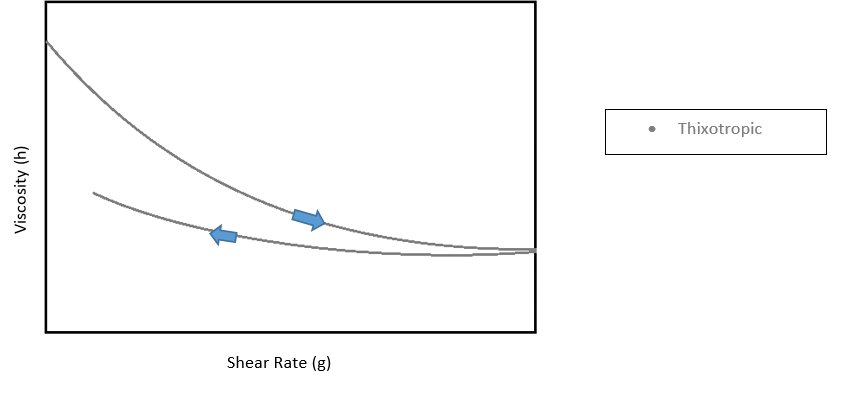
Non Newtonian fluids have a non-linear relationship between shear rate and shear viscosity. The viscosity for these fluids can be dependent not only on temperature, but also on time or deformation history (2). Most coatings and fluids are Non-Newtonian. Finding the appropriate equation to represent these fluids depends on whether it is shear thickening or shear thinning. Shear thickening materials become more viscous as the shear rate is increased, while shear thinning materials are just the opposite, becoming less viscous with the increase in shear rate. There is also a category called thixotropic. This property is identified by starting out behaving as a shear thinning fluid, then as time increases, the deformation is reversed. Paints and coatings tend to be shear thinning as they are made of many different phases that can have a desire to separate, which is an irreversible deformation. Another deformation possible under shear is the possibility of the polymer chains to go from being spiraled, or tangled, to stretched into long chains. This type of deformation is somewhat easily reversed.
Shear Rate
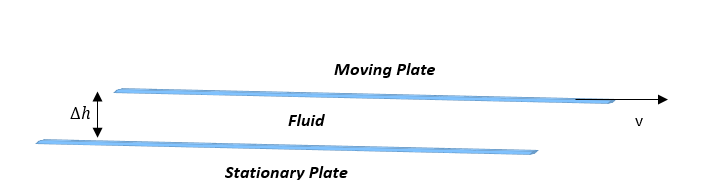
Put simply, shear rate is the speed at which the particles of a material move past each other. This is commonly demonstrated through use of the Two-Plate model. In this scenario, a fluid is placed between one stationary plate and one plate moving at a constant velocity in one direction. This velocity is then divided by the distance between the two plates to give the shear rate. Y=v/h. (3) As mentioned above, it is vital to know the shear rate of a material when calculating viscosity. A higher shear rate produces a lower viscosity, and usually means the coating is easier to be applied evenly.
Shear Stress
Shear stress is the other value needed when calculating viscosity. It can also be represented using the Two-Plate model. The movement of the upper plate causes a stress on the particles as they move past each other. This stress is defined by the force that is applied to the moving plate divided by the area of that plate. T=F/A. Shear stress combined with shear rate provide the simple equation shown above that is commonly used to calculate viscosity.
References
- Cowie, J. M. G.; Arrighi, V. Polymers: chemistry and physics of modern materials; CRC Press: Boca Raton, 2008, 345-355.
- Paul, S. Surface coatings: Science & Technology, 2nd ed.; J. Wiley: Chichester, 1997.
- Mezger, T. G. The Rheology Handbook, 2nd ed.; Vincentz Network, 2006, 19-28.