Volume 9 Issue 2
Volume 9 Issue 2
What’s Happening at Missouri S&T:
(formerly UMR)
Short Course Dates
We will be offering "Basic Composition of Coatings" September 24-28, 2012 (Fall 2012). The Basic Composition course is intended for new personnel in the coatings profession. It targets the components of coatings (resin, pigments, extenders, solvents and additives), testing and specifications, general formulation and manufacturing methods. Basic Composition is primarily a lecture course with several laboratory demonstrations.
We will be offering "Introduction to Paint Formulation" October 22-26, 2012 (Fall 2012). This course is intended to give the person a fundamental knowledge of how to approach a starting formulation and troubleshoot it. This course involves both lecture and laboratory work.
For more information see our web site at http://coatings.mst.edu and to register contact Catherine Hancock at cemv26@mst.edu or coatings@mst.edu or call 573-341-4419. **These courses are held on the Rolla Campus**
Employment Tab
We have started an employment section for our students and companies. We have a full time job section, an intern / co-op section and a graduating and alumni students section . Please explore our section on employment on our web site. Anyone wanting to have job opening listed, please contact us at (573) 341-4419 or e-mail: svgwcc@mst.edu . You can also write to us at Missouri S&T Coatings Institute, BOM #2, 651 W. 13th St., Rolla, MO 65409-1020. Our web site is http://coatings.mst.edu
Technical Insights on coatings Science
What a big difference the particle size can make:
Latex, waterborne urethane, water reducible resin
Minghang Chen, Graduate Research Student, Missouri S&T
In the waterborne coating industry, the resin can be in the form of a typical latex, polyurethane dispersion, or water reducible as three of the more common resins. Due to the different mechanisms by which they are made, there exists lots of difference among them in terms of film properties, pigmentation capacity, and particle sizes. This brief report only talks about their particle sizes different and its related effect on formulation.
1. Maximum percent solid.
All the particles immersed in liquids will adsorb more or less water on the surface. This water is often referred to as bound water. In some cases, it is also called surface water. No matter what the name is, it is generally agreed that the water will form a layer on the surface of particles which is different in many properties to bulk water. Depending on the surface properties of the particle, like HLB value, surface charge density, roughness of the particle, the thickness amount of bound water can vary from several nano meter to several tens of nano meters. 1,2 When the particle is as large as several microns, the bound water is only a small percentage of the total water. But, once the particle is as small as several tens of nanometer, or even smaller, this layer of bound water will play significant role in formulation of coatings, since a large amount of water will adhere to the surface of particles. In other words, the amount of mobile or free water is much less in nano-resins than the water in suspension with large particles. The direct consequence of the effect is that the viscosity of the suspension will increase sharply at some point where there is almost no free water. That’s why manufacture people sometimes have difficulty in increasing the percent solid of paints. Depending on the size of particle, this value (MPVF) of suspension is different. Kepler Conjecture3 suggests that the maximum packing volume fraction should be 74.05% if the particles are spherical. In reality, the value is often smaller. For example, the polystyrene latex has a value of 47~55%.4 The author has found that waterborne urethane has maximum packing volume fraction at around 45% and water reducible resin at around 37%. It shows that attentions need to put on the kind of vehicle in terms of its particle size when designing a formulation.
2. Hide of film due to particle size difference.
As to film forming, these resins will undergo a similar process. As water evaporates, the particles will approach each other. When the particles contact, the interfacial capillary force makes particle contact and begin to coalesce. When it is clear coating, where there is no pigment, the film properties are similar for all particle sizes, however, the rate of coalescence is faster as the particle size decreases. Typically a latex requires 5-10 days to coalesce while a 4 nm particle can form the film in less than 1 day. But for pigmented coating, it is a different story. Figure 15 shows the resin and pigments in two different paint systems. In figure 1.a, the system employs a water-reducible resin with particle size of 5nm as the binder, while figure 1.b uses latex with particle size 100nm. The smaller particles have more pigment content at a given volume and therefore better hide per unit film thickness.
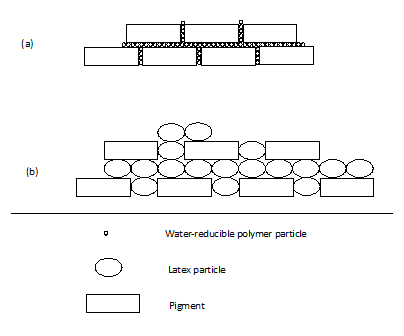
Figure 1. Resin and pigment in (a) water-reducible resin system and (b) latex system
From above, it shows that particle size of water borne resin itself can play big role in formulation. When formulating water borne paint, it is important to grasp the properties of the resin based upon its size including the amount of bound or surface water so that they are your friend, not an enemy.
Reference
(1) Palasantzas, G.; Svetovoy, V.; van Zwol, P. Physical Review B2009, 79, 1-7.
(2) Schufle, J. A.; Huang, C.-T.; Drost-Hansen, W. Journal of Colloid and Interface Science1976, 54, 184-202.
(3) Hales, T. C. Annals of Mathematics. Second Series2005, 162, 1065-1185.
(4) Pishvaei, M.; Graillat, C.; McKenna, T. F.; Cassagnau, P. Polymer2005, 46, 1235-1244.
(5) Zhao, W. Ultrasonic Microscopy Analysis of Corrosion A Nondestructive Evaluation of Paints Weathering Effects and Synthesis and Characterization of Nanoscale Water-Reducible Polymer Particles, August 2003, Missouri University of Sci & Tech (formly UMR), 2003, pp. 66-67.
