Volume 1 Issue 6
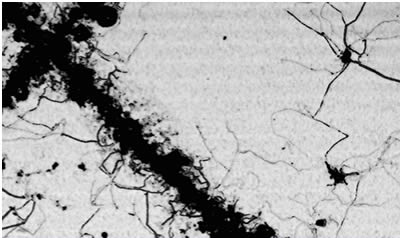
| Missouri S&T NEWS Dr. Michael R. Van De Mark We have available an Ultrasonic Microscope for doing non-destructive coatings evaluation. This instrument uses 100MHz ultrasonic sound to penetrate through a coating and view damage, corrosion, delamination or any interfacial area where sound can reflect. We have successfully followed corrosion under a coating as a function of time with good predictability. The resolution of this microscope is 0.0002 inches. The area we can scan is up to 10¿?x10¿?. Usually only one or two square inch areas are scanned. Following corrosion until a pattern develops allows the prediction of failure with good reliability. Generally we can estimate the time to failure after about 20% of the failure life has passed. This is only due to the resolution of the equipment. The technique works well over aluminum, steel, wood, plastics and many other substrates. The technique does require that the panel be submersed in water which acts to couple the sound to the panel. Air does not transmit sound very well, too much is lost. Example of filiform corrosion under a film. Corrosion was not visible except at the scribe. Technical Insights on Coatings Science Polyaniline as a corrosion inhibitor: fact or myth? Depends on the dopant. Polyaniline is a polymer synthesized via an oxidative, head-to-tail condensation of aniline monomer. Usually the synthetic oxidant of choice is potassium persulfate, which results in the chain being doped with sulfate groups and a conductive, organic metal product. The polymer is novel as high conductivities have been achieved. Applications of the polymer include electronic, sensing, and static-dissipating uses, such as in carpet fibers, utilizing natural conductive properties. However, the polymer has also been researched and marketed as a corrosion inhibitor. Use as a corrosion inhibitor has been fraught with seeming inconsistencies, despite occasional reports raving about its effectiveness. Common experience has often not met similar success. Electrochemically, a polyaniline-treated metal typically performs well but in accelerated exposures the system passivity can break down giving way to blistering or pinhole corrosion. Still there are wondrous claims. The mechanism for corrosion inhibition has been often studied, but again conflicting claims are at odds whether conductive or non-conductive forms are better, whether an overcoating is necessary, how a dopant participates, or not, in the inhibition process. We have completed studies specifically on the function of the polymer and the dopant, together and separately, with regard to the chemical structure of the dopant, and the chemical and electrochemical stability of the substrate. In our case, the substrate was chosen to be carbon steel. The coated substrates placed in corrosive environments included control epoxy coatings containing undoped polyaniline, also known as emeraldine base, doped polyaniline, dopant added to emeraldine base coating separately, or dopant alone, each system at a constant total pigment volume concentration made up with talc, and a talc control coating. The systems were studied as functions of concentration of polyaniline and dopant, acidity of the dopant through its dissociation pK, solution activity of the metal through a Pourbaix diagram, pK of the extender pigment by substituting for talc, and accelerated weathering via electrochemical polarizations, impedance spectroscopy, and salt spray. The results have been revealing. Emeraldine base is in fact an effective oxidative, anodic inhibitor, i.e., it inhibits the oxidation of the substrate by producing a passive surface oxide layer. Many have suggested the need of dopant in this function though the emeraldine base is sufficiently oxidative to perform this function alone. Unbroken films of emeraldine base in a 2-component epoxy matrix coating have provided 3000 hr. of neutral salt spray protection without blemish. However, though emeraldine base coatings can passivate steel, they do not adhere well to bare steel and has poor wet adhesion after relatively short accelerated weathering exposures. Phosphating improves the adhesion. Acidic dopants dominate the corrosion inhibition behavior. Also responsible for conductivity, dopants provide dramatically improved wet adhesion, equivalent or superior to phosphating pretreatment, of the polyaniline-in-epoxy coating under corrosive weathering exposure. The dopants can accelerate surface oxidation for electrochemical passivation but strong dopants tend to prevent normal electronic passivation as an ohmic conductor. In aqueous salt environment, the doped polymer is subject to equilibrium ion-exchange with potentially more aggressive solution anions. The strong acid dopant then serves to locally erode the passive layer and produce the often-observed localized corrosion and blistering. These observations made sense in terms of the aqueous solution stability of the metal as a function of localized pH. As the metal corrodes, dopant is consumed with formation of metal-dopant anion salts, a process previously observed as de-doping during salt fog exposure. Small polar dopant molecules aid water penetration and ion migration through the film for reduced corrosion inhibition. Soluble salts may be leached and allow continued corrosion reaction. Hydrophobic dopants, on the other hand, help prevent such transport, form stable and water resistant salts within the film, and aid protection longevity. The overcoating suggested in literature may perform a similar function to prevent or reduce the rate of solution ion-exchange. We have thus found that hydrophobic and/or weakly acidic dopants (pK~ 4 or higher), or more strongly acidic dopants at concentrations much less than required for conductivity provide for optimum corrosion inhibition results. Coatings of good tensile adhesion with unblemished, unscribed continuous salt fog (ASTM B-117) performance to 3000 hr. have been achieved, with scribed coating performance of ASTM D-1654 rating 8 at 1000 hr. exposure of cleaned and coated, but otherwise untreated cold rolled carbon steel.
Is there a topic you would like discussed? Contact us by e-mail at coatings@mst.edu. |
| September 13-17/04 Basic Composition of Coatings This course provides an overview of the components of paint and how they work. Participants are also introduced to methods for testing and manufacture of paint. |
| September 27-Oct. 1/04 Introduction to Paint Formulation This course provides techniques used in formulating paint from raw materials. It involves formulating and making paint in the laboratory, "Hand on!" |
| Coatings for Engineers available on-line anytime This course is designed to educate engineers in coatings science. Coatings systems will be covered from cleaning and surface prep to pretreatment, priming and topcoats. Specification and testing sections will aid all engineers who are charged with these tasks. |
To subscribe/unsubscribe to this newsletter, click here. Feel free to forward to this your colleagues |