Volume 12 issue 4
What’s Happening at Missouri S&T (formerly UMR):
Short Course Dates
We will be offering "Introduction to Paint Formulation" August 8-12 (Fall 2016). This course is intended to give the person a fundamental knowledge of how to approach a starting formulation and troubleshoot it. This course involves both lecture and laboratory work.
For more information see our web site at http://coatings.mst.edu and to register contact us at mstformulation@mst.edu or call 573-341-4419. **These courses are held on the Rolla Campus**
Online Short Course
We are offering "Introduction to the Coating Systems" online short course. This course is targeted for automotive and aviation type OEM companies. This self-paced seminar will cover the painting system from the composition of paints to the evaluation of the dry film. The pigments, resin, solvents and additives will be discussed including their influence on the coatings performance. Color measurement, surface profile, and other evaluation criteria will be related to composition. The importance of surface preparation and other manufacturing criteria will show the system complexity and each step's importance.
We are offering "Surface Defects: Elimination from Human and Process Contaminants" online short course. This course addresses many of the issues in prevention and minimization of defects. The course covers the defects caused by the coatings process, as well as human issues, including personal care product causes. Several of the surface defects are discussed – from basic principles and real world automotive and aircraft examples. The highly practical approach of this course will greatly aid the personnel involved in the painting operation to reduce and systematically approach issues.
Employment Tab
We have started an employment section for our students and companies. We have a full time job section, an intern / co-op section and a graduating and alumni students section . Please explore our section on employment on our web site. Anyone wanting to have job opening listed, please contact us at (573) 341-4419 or e-mail: mstformulation@mst.edu . You can also write to us at Missouri S&T Coatings Institute, BOM #2, 651 W. 13th St., Rolla, MO 65409-1020. Our web site is http://coatings.mst.edu
Technical Insights on coatings Science
Powder Coating
Peng Geng, Missouri S&T Coating Institute
Powder coating has many advantages, such as low energy and reduced resource consumption, environmentally friendly, easy processing, hard and durable coating surface, recyclable and easy to achieve automation. It has the potential to take the place of many traditional liquid coatings in the future.
Powder coating is a powder mixture which contains resin, pigment, filler, additives and etc. and having been homogenized through an extrusion and particle reduction method. The particles are very homogeneous and have a consistent particle size of typically 30-40 micron. The main film forming resin can be a single resin with its own curing system, or the combination of some different resins. Since the 1920s and 1930s, scientists began to apply polyethylene powder to metal substrates by using a flame spraying method. That is the beginning of thermoplastic powder coating[1].
In 1952, the researcher Erwin Gemmer from German company Knaspark Griesheine invented the Fluidized Bed System, which highly improved the thermoplastic powder coating. In the 1950s, most of the powder coatings were thermoplastic, and were used for pipeline, anticorrosive and electrical insulating[2].
In 1962, the Sames company came up with an electrostatic powder system. In 1964 with the development of thermosetting epoxy powder, powder coating started to draw the attention worldwide[3].
When the two oil crisis in the 1970s happened, powder coatings developed very fast since it was low energy and resource consuming, environment friendly and has high industrial efficient. Since decorative powder coating has been developed, it has become very popular with market increases of 15%-20% per year[4].
There are two kinds of powder coating, thermoset and thermoplastic. The major difference between them is in the reprocess ability of the coatings. Thermoset coatings cannot melt after the curing temperature has been reached, which means as long as the irreversible chemical boding process has not progressed it can be reheated. However when heated to the cure temperature the polymer crosslinks and the powder will not re-melt. In this case, thermoset coatings are ideal for applying to places where the more durability is needed or solvent resistance. Thermoplastic coatings have better flexibility and can be reshaped and recycled again and again making them easy to recover overspray and reuse or even change the color..
The glass transition temperature (Tg) of the powder is important and critical. It directly relates to the storage and transportation condition, as well as the producion and application condition. Tg of the polymer determines the heat resistance and the physical properties. Glass transition temperature is when the amorphous polymer starts to transitions from a hard glassy material to become soft, rubbery state.
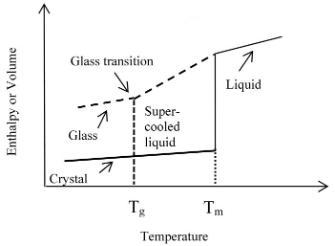
Fig 1, enthalpy or volume of various states of material affected by temperature [5].
As it is shown in fig 1, when the temperature is below Tg, the polymer chain movement is frozen, the structure of the molecule is stable at its molecular random state. If the temperature is increased to above the Tg, the polymer chains starts to move creating free volume which allows the chains to slowly move or flex.
The stability of the powdered coating primarily depends on the composition of the resin. The glasss transition temperature of a polymer is dependent upon the molecular weight of the resin. The equation is given.
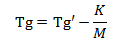
Tg’ is the temperature when molecular weight is infinity;
Mn is the number average molecular weight; K is the constant.
The higher molecular weight has an even more important role in that it decreases the diffusion coefficient of the polymer and thus increases the viscosity slowing the flow and leveling of the powder coating. If the viscosity is too high, it will yield poor flow and orange peel will be observed. According to the William-Landel-Ferry equation, when the temperature is relatively low, around the polymer’s glass transition temperature, Tg ˂ T ˂ Tg + 100 ºC, the viscosity of polymer increases very fast with the decreasing of the temperature[6].
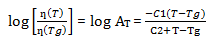
ƞ(Tg) is the viscosity of at Tg; C1 and C2 are constants[7].
So for powder coating polymer, it is important to ensure that the powder solid must be stored at the temperature lower than its Tg, for both the mechanical properties of the powder, as well as the stability during storage and transportation. Commonly, the Tg has to be at least 50 ºC. If you would like to make the Tg lower, the coating will need to be kept cool during both transportation and storage.
During manufacturing the powdered ingredients are mixed and run through a twin screw extruder. This process disperses the pigments 100%. The temperature in the extruder must be kept below the cross linking temperature or the extruder will sease up. The chilled extrudate is then powdered to produce a powder coating. The fines cannot generally be returned or reworked due to the fact that some surfaces in the extruder cause some cross linking and reprocessing would increase the viscosity too much resulting in poor flow and le3veling Thus, the curing temperature will have to be higher than the extruder temperature[8], which is usually between 140 ºC to 220 ºC.
Reference
1. Harry, O. D. (1920). Means for applying coating. US1332544 A.
2. Emerson, B. F. (1977). Thermoplastic powder coating systems. US 4211691 A.
3. George, A. B. (1962). Method and apparatus for coating metal strip and wire. US 3019126 A.
4. Agblor, K. (2006). The Cropportunity Strategy for Red Lentils-Spot Light on Research. PulsePoint. June, pp.25-27: Saskatchewan Pulse Growers.
5. Debenedetti, P. G. & Stillinger, F. H. (2001). Supercooled liquids and the glass transition. Nature 410, pp 259-267.
6. Joel, R. F. Polymer Science And Technology, third edition, pp 451-452.
7. Malcolm, L. W; Robert, F. L & John, D. F. (1955). The Temperature Dependence of Relaxation Mechanisms in Amorphous Polymers and Other Glass-forming Liquids. J. Am. Chem. Soc, pp 3701-3707.
8. X, Ramis; A, Cadenato; J.M, Morancho & J.M, Salla. (2003). Curing of a thermosetting powder coating by means of DMTA, TMA and DS. Polymer. March, pp 2067-2079.
